Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Different jakinibs have shown efficacy in rheumatoid arthritis (RA) but in an important proportion of patients, insufficient response leads to therapy withdrawal. The different jakinibs show variable selectivity for the four Jak isoforms (Jak1,2,3 y Tyk2) but there are no clinical trials analyzing the response to a jakinib after the suspension of another jakinib and therefore, observational data may be useful in this regard. The aim of this study is to describe efficacy and safety of the second jakinib in patients with suspension of the first due to failure or side effects.
Methods: Spanish observational multicentric study. Data were retrospectively obtained from medical records of 31 patients with RA sequentially treated with baricitinib or tofacitinib in any order.
Results: We identified 31 patients with RA, median age 62 years (IQR 51-67), 80.6% female (Table 1). Rheumatoid factor and anti-CCP was positive in 80.6% and 71% of the patients . Most of the patients (87%) had received previously treatment with bDMARD, median number of previous bDMARDs 4 (IQR 2-5). Half of the patients received Tofacitinib first, and the other half Baricitinib as the first Jakinib. Median survival for the first Jakinib was 5 months (IQR 3-8) and the reason for withdrawal was inefficacy in 19 cases (61.3%) and adverse effects in 12 (38.7%). Median DAS28CPR in the beginning of the second jakinib was 5.3 (IQR 5-5.9), 9 patients were treated in monotherapy and 26 used glucocorticoids.
9 patients discontinued the second Jakinib, in all of them the reason was inefficacy. The treatment suspension rate was similar among patients discontinuing the first Jakinib for inefficacy (5/19, 26.3%) or for adverse effects (3/12; 25%). Median follow-up of patients who didn’t discontinue the second Jakinib was 19.5 (IQR 12-24) months. Disease activity data along this follow-up are depicted in Figure 1 and 2.
Conclusion: Our data show that therapy with a second jakinib is a safe and efficacious option after discontinuation of the first jakinib due to either inefficacy or side effects. The response rate to the second jakinib is similar in patients with inefficacy or side effects which suggests that failure to the first does not reduce the chance of response to the second.
Acknowledgements: M. Retuerto was recipient of a training grant from Sociedad Española de Reumatología (SER).
 Table 1. Characteristics of 31 patients with RA treated sequentially with two jakinibs.
Table 1. Characteristics of 31 patients with RA treated sequentially with two jakinibs.
 Figure 1. Second jakinib treatment results during the follow-up period (median).
Figure 1. Second jakinib treatment results during the follow-up period (median).
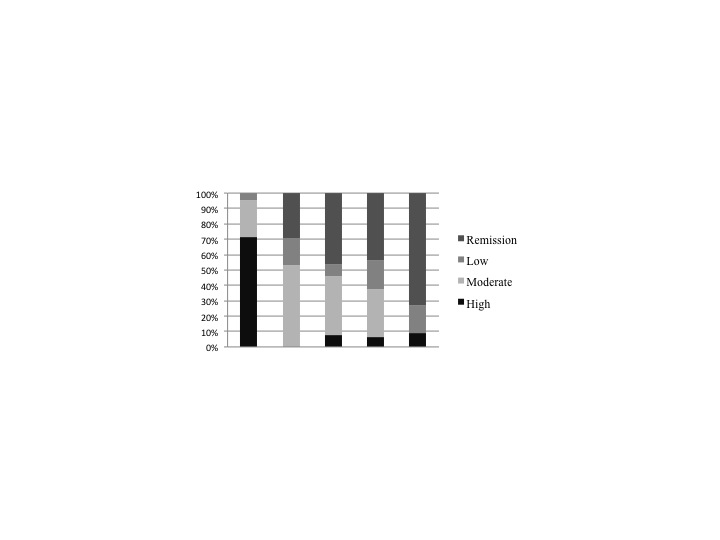 Figure 2. Second jakinib activity development.
Figure 2. Second jakinib activity development.
To cite this abstract in AMA style:
Retuerto M, Trujillo E, Valero C, Fernández-Espartero C, Soleto-Kharkovskaya C, García Valle A, Aurrecoechea E, Garijo M, Loricera J, Pablos J. Efficacy and Safety of Switching Jakinibs in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-switching-jakinibs-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-switching-jakinibs-in-rheumatoid-arthritis/
