Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Intravenous (IV) anifrolumab (300 mg, every 4 weeks [Q4W]) is an approved biologic add-on therapy for moderate to severe SLE;1 a subcutaneous (SC) formulation may allow for greater personalization of treatment. Here, we report interim results from a global phase 3 study evaluating the efficacy and safety of SC anifrolumab in patients with moderate to severe SLE.
Methods: In TULIP-SC (NCT04877691), eligible adult patients with SLE (revised 1997 ACR criteria) were randomized 1:1 to receive weekly (QW) anifrolumab 120 mg SC or placebo plus standard therapy during a 52-week double-blind treatment period. This pre-planned interim analysis (IA) of 346 patients was conducted once 220 patients completed the double-blind treatment period or discontinued.Efficacy was assessed in the IA set (n=220); the safety set included all randomized patients who received ≥1 dose at time of IA data cut-off (n=346). The difference between groups in the proportions of BILAG-based Composite Lupus Assessment (BICLA) responders (primary endpoint; stratified Cochran-Mantel–Haenszel approach) was assessed at Week 52. Key secondary endpoints (proportions of BICLA responders whose oral corticosteroid [OCS] dose from Week 40 through Week 52 was ≤7.5 mg/day if baseline [BL] was ≥10 mg/day or ≤BL if BL was < 10 mg/day, time to first BICLA response sustained to Week 52, and time to first flare) and the proportion of patients achieving SLE Responder Index response (SRI[4]) at Week 52 were also assessed.
Results: Baseline demographics and key clinical characteristics were balanced between groups (anifrolumab: n=109, mean [standard deviation] age=43.0 [12.4] years, SLEDAI-2K score=11.3 [3.8]; placebo n=111, mean age=42.3 [11.4] years, SLEDAI-2K score=10.5 [2.9]), with similar discontinuation (21.1% vs 21.6%).The primary endpoint was met: 60.3% vs 43.9% of patients achieved a BICLA response at Week 52 with anifrolumab vs placebo, respectively (Figure 1; difference [95% CI]=16.5% [3.3–29.6%]; p=0.014). At Week 52, the proportion of BICLA responders who met the OCS dosing criteria, and the proportion achieving SRI(4) were numerically greater with anifrolumab vs placebo (Figure 1). Patients receiving anifrolumab vs placebo were around twice as likely to achieve a BICLA response sustained through Week 52 (Figure 2). The risk of flare over 52 weeks with anifrolumab vs placebo was numerically lower (HR [95% CI]=0.69 [0.4–1.1]; nominal p=0.093). The incidence of adverse events (AEs) was 74.4% (131/176) vs 71.2% (121/170) with anifrolumab and placebo treatment, respectively; similar proportions of serious AEs (10.8% vs 8.8%) and serious infections (6.3% vs 3.5%) were reported. Injection site reactions (ISR) occurred in 15.3% vs 17.1% with anifrolumab and placebo treatment, respectively. Most AEs, including ISRs, were of mild to moderate intensity.
Conclusion: Treatment with anifrolumab 120 mg SC QW provided a statistically significant and clinically meaningful benefit in overall disease activity over standard therapy and was well tolerated; the efficacy and safety profile was consistent with the approved IV 300 mg anifrolumab treatment regimen.1 1. Saphnelo Prescribing Information. http://www.azpicentral.com/pi.html?product=saphnelo.
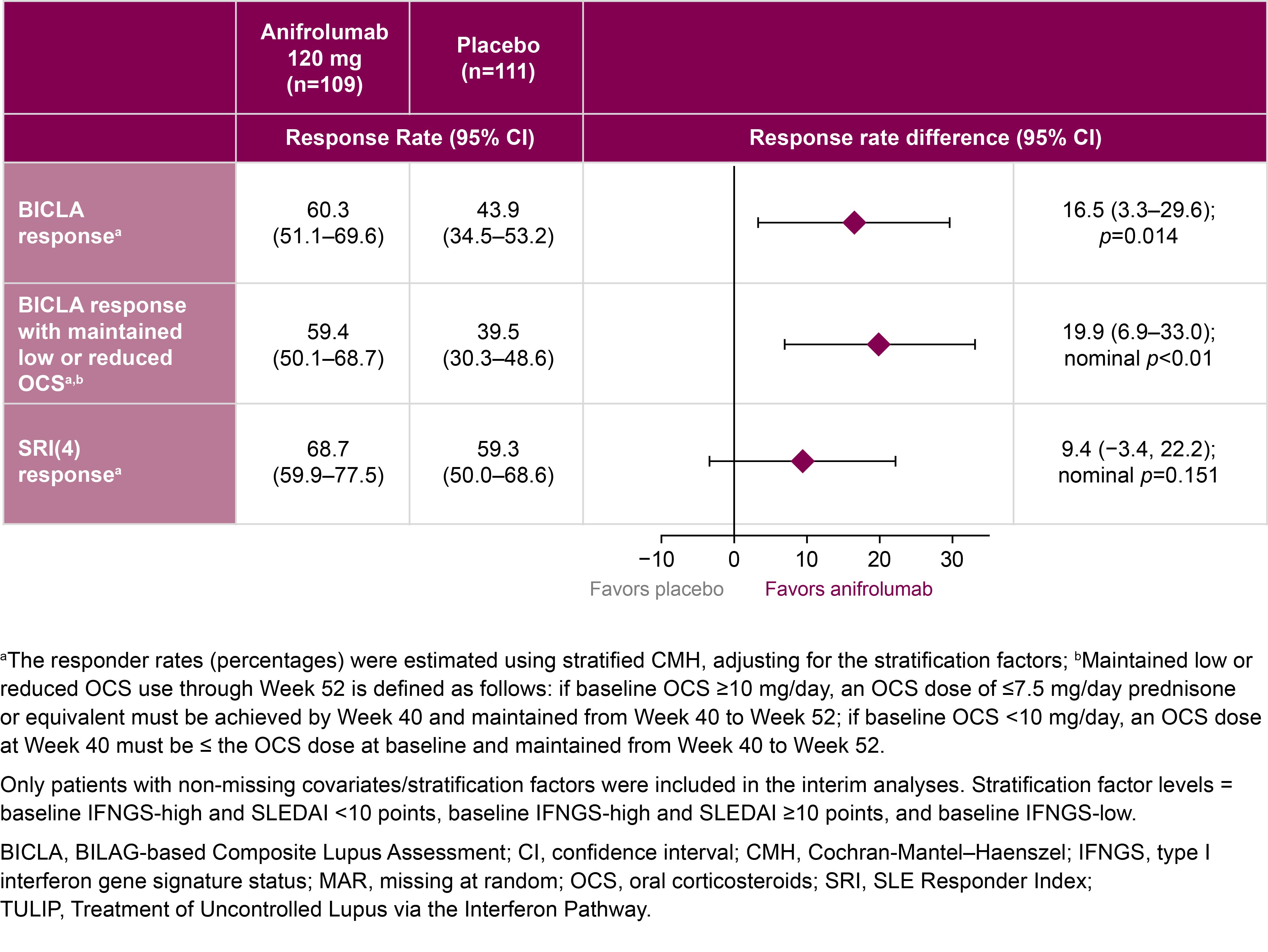 Figure 1: Interim efficacy results at Week 52 of the phase 3 TULIP-SC trial of weekly subcutaneous anifrolumab alongside standard therapy
Figure 1: Interim efficacy results at Week 52 of the phase 3 TULIP-SC trial of weekly subcutaneous anifrolumab alongside standard therapy
.jpg) Figure 2: Time to first BICLA response sustained up to, and including, Week 52 during the phase 3 TULIP-SC trial of weekly subcutaneous anifrolumab alongside standard therapy
Figure 2: Time to first BICLA response sustained up to, and including, Week 52 during the phase 3 TULIP-SC trial of weekly subcutaneous anifrolumab alongside standard therapy
To cite this abstract in AMA style:
Manzi S, Bruce I, Morand E, Furie R, Tanaka Y, Puzio P, Khan E, Wissmar J, Song M, Lindholm C. Efficacy and Safety of Subcutaneous Anifrolumab in Systemic Lupus Erythematosus: Interim Analysis of a Phase 3 Randomized Placebo-controlled Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-subcutaneous-anifrolumab-in-systemic-lupus-erythematosus-interim-analysis-of-a-phase-3-randomized-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-subcutaneous-anifrolumab-in-systemic-lupus-erythematosus-interim-analysis-of-a-phase-3-randomized-placebo-controlled-study/
