Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: In this study, we aimed to evaluate the results of secukinumab treatment in patients with Axial Spondyloarthritis (AxSpA) who were enrolled in the TURKBIO cohort.
Methods: In our study, AxSpA patients registered in the TUKRBIO database were evaluated. 6, 12 and 24 months of secukinumab drug retention, inactive disease/low disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] < 2/< 4, Ankylosing Spondylitis Disease Activity Score [ASDAS] < 1.3/< 2.1) and response rates (BASDAI50, Spondyloarthritis International Society [ASAS] 20/40, ASDAS clinically significant improvement [ASDAS-CII] and ASDAS major improvement [ASDAS-MI]) were evaluated.
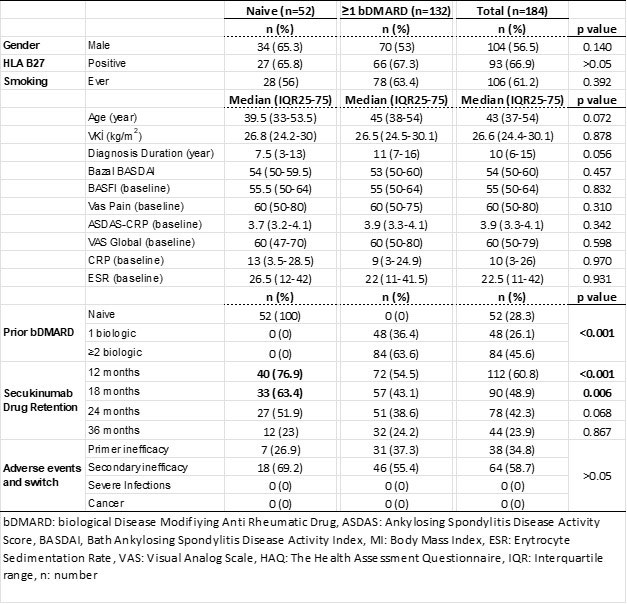
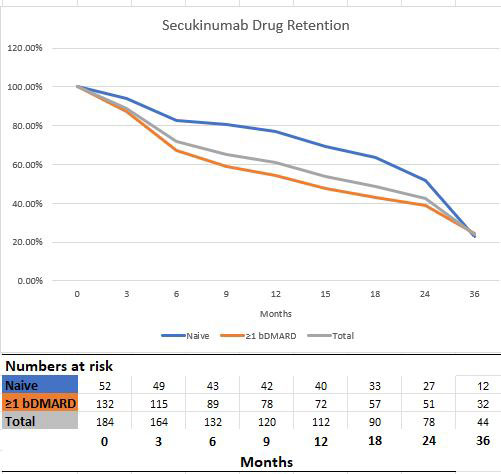
Results: Follow-up data of 184 AS patients registered in the TURKBIO registry were evaluated. Of these patients, 52 (28.3%) were bDMARD naive patients and 132 (71.7) had used at least one bDMARD prior to secukinumab. Age and gender distribution were similar between bDMARD naive and ≥1 bDMARD groups. At 1-year follow-up, 60 and 12 patients discontinued their treatment in the ≥1 bDMARD group and the secukinumab group, respectively (p< 0.001). Ineffectiveness was the most common reason for discontinuing treatment in the ≥1 bDMARD and secukinumab groups. The rate of continuation of secukinumab treatment was 71.7% at 6 months and 60.9% at 12 months. When clinical conditions were assessed based on prior bDMARD use, there were significantly better drug survival rates at 6 months and 12 months for the bDMARD naive group (82.7% and 76.9 months, respectively). In addition, it was determined that the symptoms and the time elapsed after diagnosis did not affect the efficacy of secukinumab. In our study, no significant drug safety problem was encountered in the follow-up of secukinumab treatment for more than 3 years.
Conclusion: In a study conducted with TURKBIO real-life data, we found that secukinumab treatment in patients with AxSpA had better clinical response and higher drug survival rates in biologically naive patients, similar to TNFi treatment. However, it has been observed that the use of secukinumab in the second or subsequent steps in patients who do not respond to biologics has similar success rates to TNFi treatment results.
To cite this abstract in AMA style:
Gulle S, Karakas A, Can G, Senel S, Capar S, dalkilic H, Akar S, Koca S, Tufan A, Yazici A, Yilmaz S, Inanc N, Birlik M, Solmaz D, Cefle A, Goker B, Yolbas S, Krough N, Yilmaz N, Erten S, Bes C, Soysal O, Ozturk M, Haznedaroglu S, Yavuz S, Direskeneli H, Onen F, Sari I. Efficacy and Safety of Secukinumab in the Treatment of Axial Spondyloarthritis: Real-Life Data from TURKBIO Cohort [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-secukinumab-in-the-treatment-of-axial-spondyloarthritis-real-life-data-from-turkbio-cohort/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-secukinumab-in-the-treatment-of-axial-spondyloarthritis-real-life-data-from-turkbio-cohort/