Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Use of JAK inhibitors (JAKi) may be challenged in difficult to treat rheumatoid arthritis (D2TRA) by the multiplicity of previous treatment lines and the presence of comorbidities that may trigger the occurrence of potentially severe side effects. Our objective was to assess the efficacy and safety profile of JAKi in patients with D2TRA in clinical practice
Methods: Retrospective routine care study carried out between 2018 and 2022 our department. We selected from our electronic medical report database RA patients initiating a JAKi between 2018 and 2022. D2TRA was defined by failure to at least two targeted biological therapies of different mechanisms of action and at least one of the following: active disease, defined by a DAS28 >3.2, signs and/or symptoms suggestive of active disease or inability to taper glucocorticoid treatment below 7.5mg/day (1). D2TRA patients were compared to JAKi-treated patients not fulfilling this D2TRA definition (non-D2TRA). Efficacy was assessed at the first visit (FV) (3 to 6 months following the JAKi initiation) and at the last available visit (LV) up to December 2022 on the basis of the DAS28 and DAS28-CRP composite index and its components. The number of side effects and causes of treatment discontinuations were collected during the exposition period
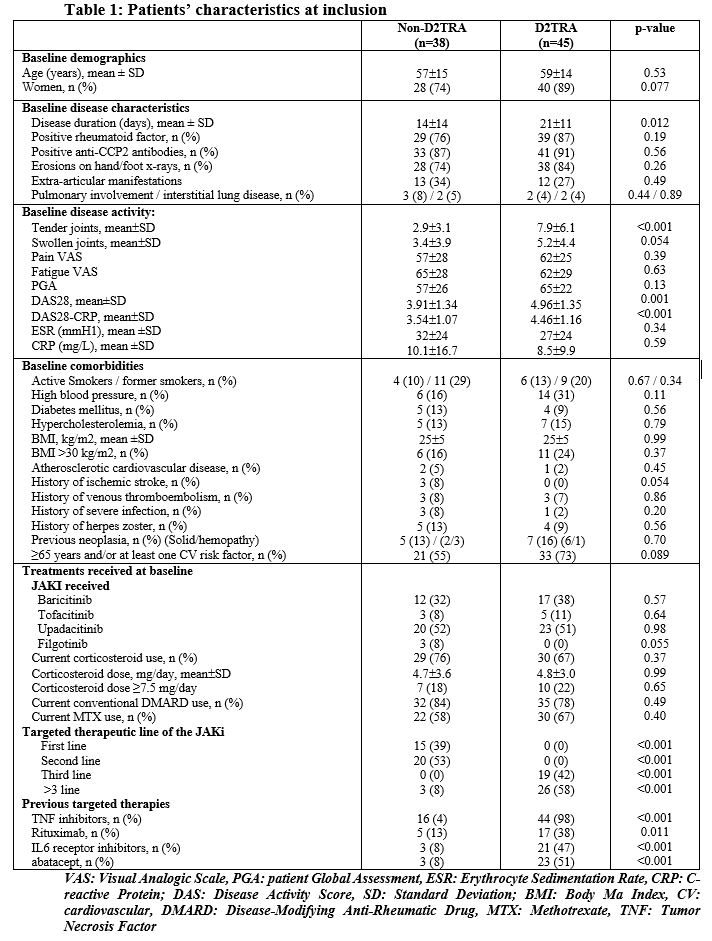
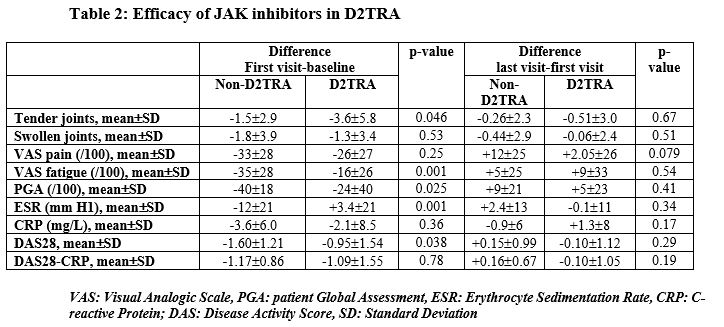
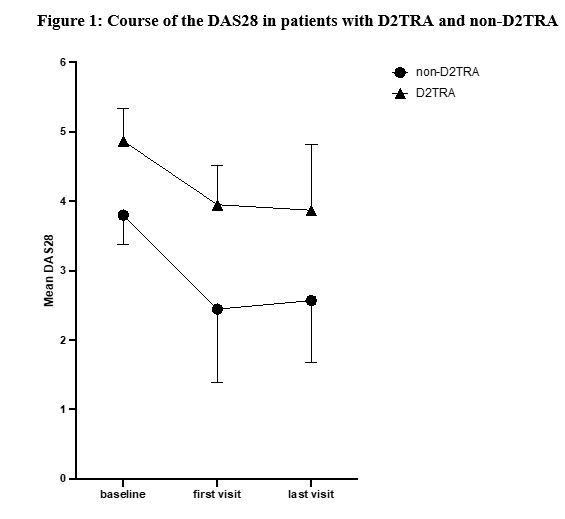
Results: We included 83 RA patients initiating a JAKi, with a mean age of 58±14 years and a mean disease duration of 16±13 years. Among them, we identified 45 D2TRA and 38 non-D2TRA patients (Table 1). Patients with D2TRA had longer disease duration and active disease and previous targeted therapies. DAS28 was reduced between baseline and FV in both groups, with a significantly higher extent in non-D2TRA patients, and then remain stable in both groups between FV and the LV, which occurred 15±10 months after the baseline (Table 2). These results were similar for other parameters assessing disease activity (Table 2). Higher proportion of patients reached remission or low disease activity (LDA, DAS28< 3.2) in the non-D2TRA subgroup (FV: 82% vs. 53%, p=0.012; LV: 71% vs. 33%, p=0.006) compared to D2TRA. A total of 35 patients discontinued the JAKi during a mean observation period of 20±10 months. Mean time to discontinuation was 10±8 months. Frequency of discontinuation was not different for D2TRA and non-D2TRA patients (21/45, 47% vs 14/38, 37%, p=0.36). Discontinuations related to inefficacy and side effects occurred in 20/83 patients (24%) and 11/83 patients (13%), respectively, and were evenly distributed between patients with D2TRA and non-D2TRA. Frequency of infections (n=31), herpes zoster (n=3) myocardial infractions (n=3) and venous thromboembolism (n=3) was similar between groups. These events were more likely to occur in the subgroup of patients aged ≥65 years and/or with at least one CV risk factor (30/39, 77%)
Conclusion: JAKi reduced disease activity parameters of patients with D2TRA. However, disease activity level of these patients remained high, with a proportion of LDA or remission significantly lower compared to non-D2TRA, highlighting the need of improved therapeutic strategies in D2TRA. Tolerance profile of JAKi was not different between patients with D2TRA and non-D2TRA and largely depended on the presence of risk factors
To cite this abstract in AMA style:
AL TABAA O, HECQUET S, THOMAS M, CARVES S, Combier A, MICELI C, Fogel O, Molto A, ALLANORE Y, AVOUAC J. Efficacy and Safety of JAK Inhibitors in Difficult to Treat Rheumatoid Arthritis in Clinical Practice [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-jak-inhibitors-in-difficult-to-treat-rheumatoid-arthritis-in-clinical-practice/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-jak-inhibitors-in-difficult-to-treat-rheumatoid-arthritis-in-clinical-practice/