Session Information
Date: Wednesday, November 8, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment V
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Ixekizumab (IXE) is a high affinity monoclonal antibody that selectively targets interleukin-17A. In patients with active psoriatic arthritis (PsA) who had an inadequate response to tumor necrosis factor inhibitors (TNFi), IXE was superior to placebo (PBO) in improving the signs and symptoms of PsA after 24 weeks of treatment (SPIRIT-P2; NCT02349295).1 The objective of this study is to report the Week 52 interim efficacy and safety findings of IXE treatment during the Extension Period (EP) of SPIRIT-P2 (Weeks 24-156).
Methods: SPIRIT-P2 is a phase 3, multicenter, double-blind study. All 363 patients had an inadequate response to one or two TNFi or were intolerant to TNFi. During the Double-Blind Treatment Period (DBTP; Weeks 0-24), patients were randomly assigned 1:1:1 to subcutaneous administration of either 80 mg IXE every 4 weeks (Q4W; N=122) or every 2 weeks (Q2W; N=123) following a 160 mg starting dose at Week 0, or PBO (N=118). Of these, 310 patients completed the DBTP and entered the EP (Weeks 24-156). Patients randomized to IXE at Week 0 continued the same dose regimen in the EP. PBO patients were re-randomized (1:1) to IXE Q4W or Q2W at Week 16 (inadequate responders) or 24. In this interim analysis, efficacy (up to Week 52) and safety (up to Week 156) were analyzed using the EP population, defined as all patients who received at least 1 dose of study drug in the EP. Missing values were considered non-response for categorical data and were imputed by modified baseline observation carried forward for continuous data.
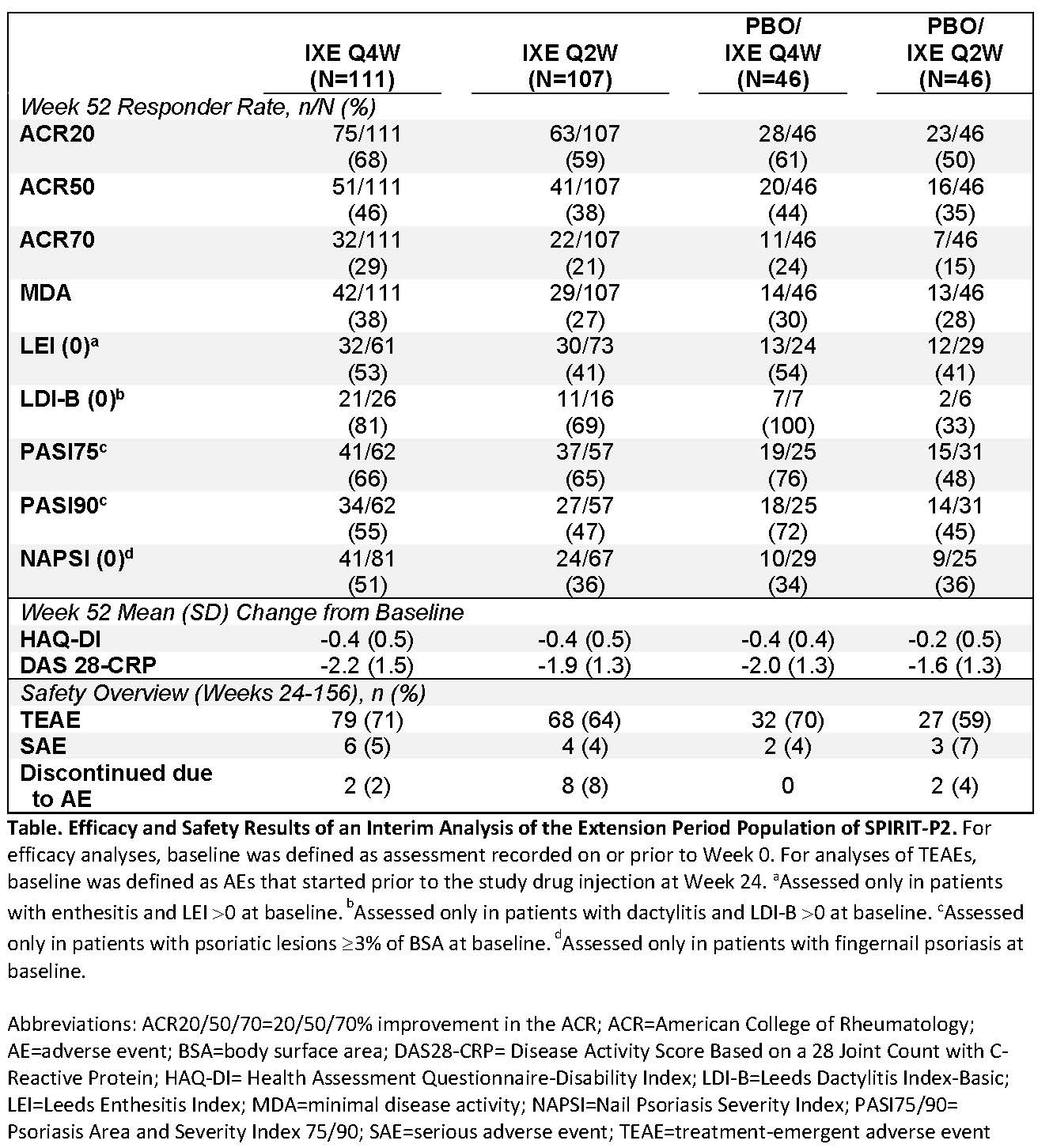
Results: In the DBTP, a significantly higher percentage of patients achieved ACR20 at Week 24 with IXE Q4W (53%) or Q2W (48%) than with PBO (20%).1 For patients who entered the EP, the mean age was 52 years, 47% were male, the mean time since PsA onset was 12 years, and mean tender and swollen joint counts at baseline (Week 0) were 23 and 12, respectively. For EP patients who were initially randomized to IXE Q4W or Q2W during the DBTP, ACR20 responses at Week 52 were 68% and 59%, respectively. For patients treated with PBO during the DBTP and re-randomized to IXE Q4W or Q2W during the EP, ACR20 responses at Week 52 were 61% and 50%, respectively. Additional efficacy measures are depicted in the Table. The frequency of adverse events (AEs) in the EP are presented in the Table; the majority were mild or moderate in severity. Serious AEs occurred in 15 patients, and one death occurred in the EP population: a myocardial infarction in a PBO/IXE Q2W patient 502 days after starting IXE.
Conclusion: IXE demonstrated sustained improvement in the signs and symptoms of PsA across treatment groups during the EP. The safety profile of IXE observed in the EP population was consistent with the safety profile of the intent-to-treat population in the DBTP of SPIRIT-P2.1
Reference: 1. Nash P et al. Lancet. 2017. E-pub ahead of print 24 May, 2017. http://dx.doi.org/10.1016/S0140-6736(17)31429-0
To cite this abstract in AMA style:
Genovese MC, Combe B, Kremer J, Adams D, Lee C, Kerr L, Nash P. Efficacy and Safety of Ixekizumab in Patients with Active Psoriatic Arthritis and Previous Inadequate Response to TNF Inhibitors: 52-Week Results from a Phase 3 Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-ixekizumab-in-patients-with-active-psoriatic-arthritis-and-previous-inadequate-response-to-tnf-inhibitors-52-week-results-from-a-phase-3-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-ixekizumab-in-patients-with-active-psoriatic-arthritis-and-previous-inadequate-response-to-tnf-inhibitors-52-week-results-from-a-phase-3-study/