Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Juvenile psoriatic arthritis (JPsA) and enthesitis-related-arthritis (ERA) are 2 categories of Juvenile Idiopathic Arthritis (JIA) according to the International League of Associations for Rheumatology classification and represent analogous pediatric forms of adult psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), respectively. Ixekizumab (IXE), an anti-interleukin-17A monoclonal antibody, has demonstrated efficacy and safety in adults with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA). This study evaluated efficacy and safety of IXE in paediatric patients with active Juvenile psoriatic arthritis (JPsA) and enthesitis-related arthritis (ERA) through week (W) 16.
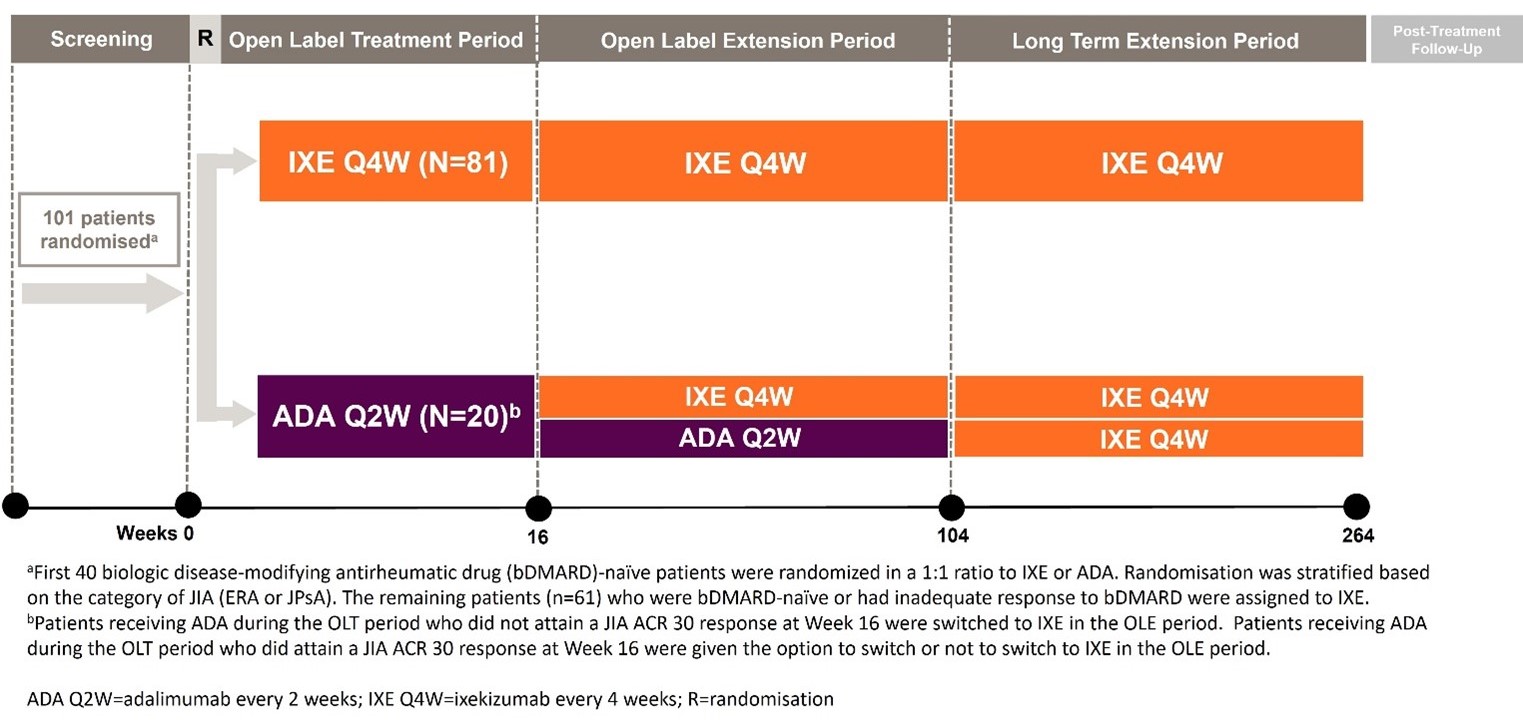
Methods: COSPIRIT-JIA is an on-going multicentre, randomised, open-label Phase 3 study of IXE, with adalimumab (ADA) as a reference arm in patients aged 2 – < 18 years with active JPsA/ERA. It is conducted in 3 periods: 16W open-label treatment (OLT) period followed by open-label extension (OLE) period proceeding to W104, followed by an open-label long term extension period (LTE) out to a total of 264W (Figure1). Patients received either subcutaneous IXE or ADA based on weight during the OLT and OLE period (IXE every 4 weeks (Q4W): 20mg (starting dose 40mg) for patients 10 – < 25kg; 40mg (starting dose 80mg) for patients 25 – 50kg and 80mg (starting dose 160mg) for patients >50kg; and ADA every 2 weeks (Q2W): 20mg for patients 10 – < 30kg and 40mg for patients ≥30kg). At the end of OLE, all remaining ADA patients will be transitioned to IXE or discontinued. Primary endpoint: IXE-treated patient percentage meeting the JIA American College of Rheumatology (ACR) 30 response criteria at W-16.
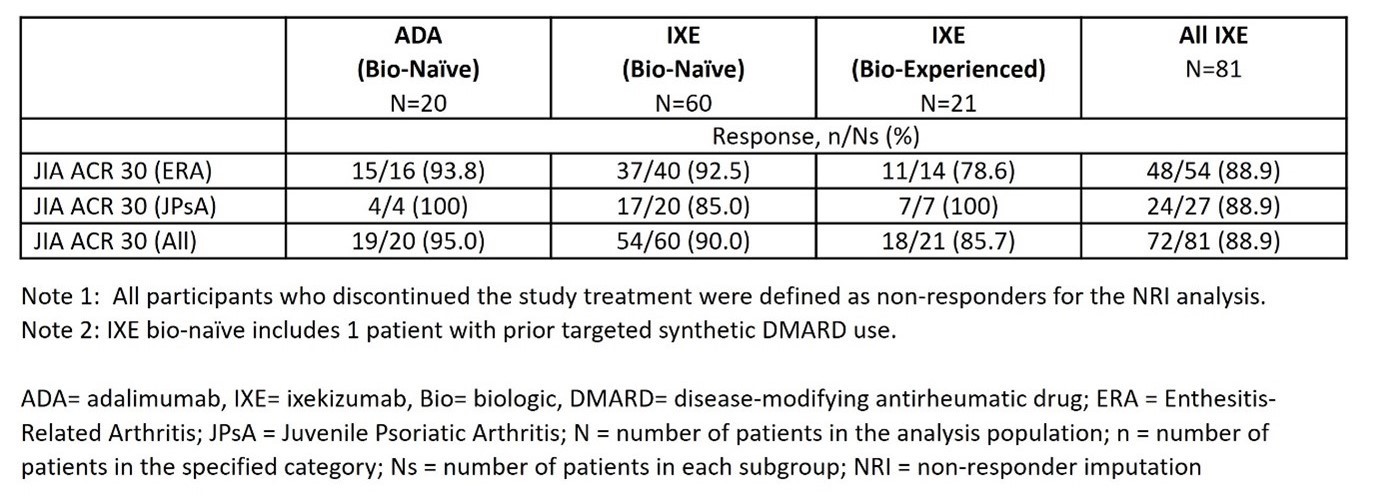
Results: Total 101 patients (IXE=81, ADA=20) were enrolled into the 16-W OLT period. At baseline, the study population included 44 (43.6%) females, had mean age of 13.1(±3.1) years, total Psoriasis Area and Severity Index score 4.4±3.0 and Leeds Enthesitis Index 2.1± 1.1. Patients were diagnosed with JPsA (31 [30.7%]) and ERA (70 [69.3%]). At the end of OLT, 72 (88.9%) of all IXE-treated patients achieved JIA ACR30 (Table 1). Response rates were similar across IXE-treated bio-naïve (54 [90.0%]) and bio-experienced (18 [85.7%]) patients and across ERA (48 [88.9%]) and JPsA (24 [88.9%]) categories (Table 1). In the OLT period, 81.5% IXE-treated patients presented treatment-emergent adverse events (most were mild). Serious adverse events: reported by 3.7% IXE-treated patients. No new safety signals were observed.
Conclusion: In paediatric patients with JPsA and ERA, efficacy of IXE was demonstrated with an overall 88.9% JIA ACR30 response rate at W16. Safety findings were consistent with the known safety profile of IXE.
To cite this abstract in AMA style:
Ramanan A, Ruperto N, Foeldvari I, Vega Cornejo G, Keller S, Wang R, Araújo J, Hojnik M, Sen P, Marulkar K, QUARTIER P. Efficacy and Safety of Ixekizumab in Children with Active Juvenile Psoriatic Arthritis and Enthesitis Related Arthritis (COSPIRIT-JIA): 16-week Results of a Multicentre, Randomised, Open-label Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-ixekizumab-in-children-with-active-juvenile-psoriatic-arthritis-and-enthesitis-related-arthritis-cospirit-jia-16-week-results-of-a-multicentre-randomised-open-label-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-ixekizumab-in-children-with-active-juvenile-psoriatic-arthritis-and-enthesitis-related-arthritis-cospirit-jia-16-week-results-of-a-multicentre-randomised-open-label-study/