Session Information
Date: Wednesday, November 13, 2019
Title: 6W010: Pediatric Rheumatology – Clinical III: Juvenile SLE & Dermatomyositis (2864–2869)
Session Type: ACR Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Belimumab (BEL) is the first treatment approved for children with systemic lupus erythematosus (SLE) from 5 years of age and older.1 This approval was based on the PLUTO trial (NCT01649765), which was designed to assess the safety and efficacy of BEL in children with SLE.2 Additional evidence to support the consistency of effect of BEL in children included an across-trial comparison of efficacy and safety in adult SLE with the BLISS-52 (NCT00424476), BLISS-76 (NCT00410384), and LBS02 (safety only; NCT00071487) trials. Results are presented below.
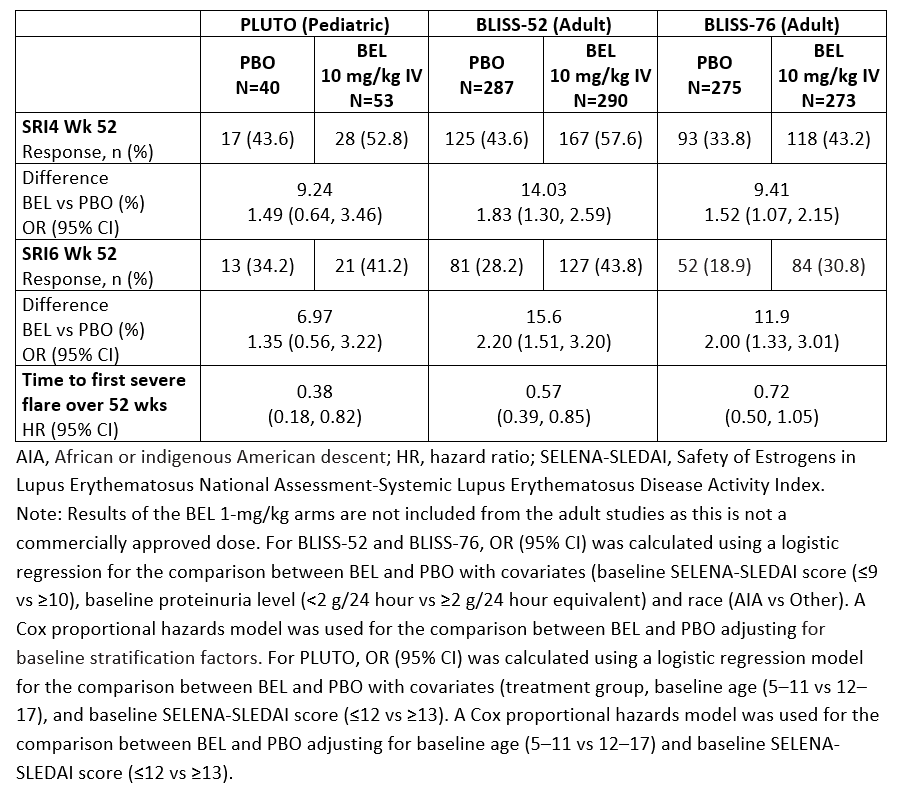
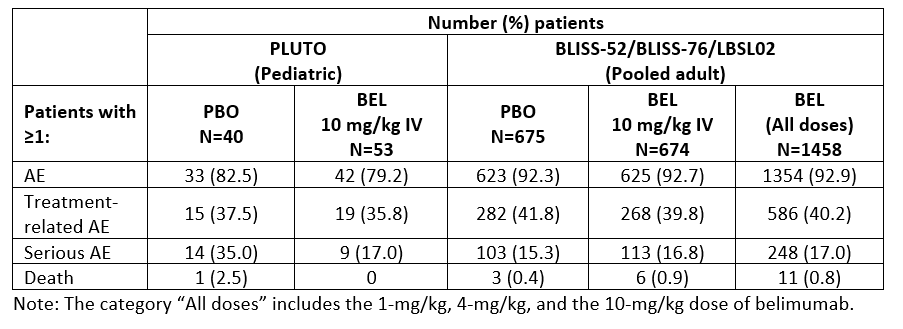
Methods: The trials enrolled patients aged 5–17 years (PLUTO) and ≥18 years (BLISS-52 and BLISS-76) with a diagnosis of SLE, and active disease and seropositivity at screening. Patients were randomized to BEL 10 mg/kg given intravenously (IV) or placebo (PBO), plus standard of care (SoC) in PLUTO; and BEL 1 mg/kg or 10 mg/kg IV or PBO, plus SoC, in the BLISS trials. The primary endpoint across these trials was the SLE Responder Index 4 (SRI4) at Week (Wk) 52. Additional endpoints included SRI6 at Wk 52 (post hoc endpoint in the BLISS trials) and time to first severe flare over 52 wks. An across-trial comparison (intent-to-treat [ITT]) of PLUTO, BLISS-52, and BLISS-76 summarizes the treatment effect of BEL IV plus SoC versus PBO plus SoC on SRI4 and SRI6 at Wk 52, reported as odds ratio (OR; 95% confidence interval [CI]), and time to first severe flare, reported as a reverse hazard ratio (95% CI). Safety analyses included adverse events (AEs), serious AEs (SAEs), and AEs of special interest (AESI) for PLUTO and pooled data from BLISS-52, BLISS-76 and LBSL02. The results of both the efficacy and safety across-trial comparisons are descriptive only.
Results: SRI4 and SRI6 responses at Wk 52 consistently favored BEL IV plus SoC. Reduction in the risk of severe flare was seen with BEL IV plus SoC versus PBO plus SoC in the PLUTO, BLISS-52, and BLISS-76 trials (Table 1). The incidences of patients experiencing ≥1 AE and ≥1 SAE in the PLUTO trial and the pooled safety data from the adult studies were similar (Table 2). With respect to AESI, there was no difference between treatment groups across the pediatric and adult studies in malignant neoplasms and post-infusion systemic reactions. The rate of all infection AESI was higher in PLUTO (PBO 7.5% and BEL 13.2%) compared with the adult studies (PBO 5.5%, BEL 10 mg/kg 4.7%), with an observed increase in herpes zoster events but no clinically meaningful differences in serious infections in PLUTO compared with the adult studies. There was no imbalance between treatment groups in depression/suicide/self-injury AESI in PLUTO, and the incidence was lower in the BEL group in PLUTO (1.9%) compared with that in the adult studies (BEL 10 mg/kg 8.8%).
Conclusion: The efficacy and safety of BEL IV plus SoC in children with SLE are consistent with those in the adult BEL study populations and support a favorable benefit/risk profile in ages 5 and older.
Study funded/conducted by GSK.
References:
1) Benlysta prescribing information https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125370s064,761043s007lbl.pdf [Accessed May 2019].
2) Brunner HI, et al. Arthritis Rheumatol. 2018;70(59):3224–5, Abst. 2867
To cite this abstract in AMA style:
Nino A, Bass D, Eriksson G, Hammer A, Ji B, Quasny H, Roth D. Efficacy and Safety of Intravenous Belimumab in Children with Systemic Lupus Erythematosus: An Across-Trial Comparison with the Adult Belimumab Studies [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-intravenous-belimumab-in-children-with-systemic-lupus-erythematosus-an-across-trial-comparison-with-the-adult-belimumab-studies/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-intravenous-belimumab-in-children-with-systemic-lupus-erythematosus-an-across-trial-comparison-with-the-adult-belimumab-studies/