Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment (0985–0989)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Iberdomide is a high-affinity cereblon ligand that promotes ubiquitination and proteasomal degradation of Ikaros (IKZF1) and Aiolos (IKZF3), transcription factors linked to the genetic risk for systemic lupus erythematosus (SLE). Efficacy, safety, pharmacodynamics (PD), and pharmacokinetics (PK) of iberdomide were evaluated in a phase 2b study in patients (pts) with active SLE (NCT03161483).
Methods: Adults (N=288) with autoantibody-positive SLE by ACR criteria and with SLEDAI 2K score ≥6 were randomized (2:2:1:2) to oral iberdomide (0.45, 0.3, 0.15 mg) or placebo (PBO) daily for 24 wks, on standard background medications. Pts on PBO were re-randomized to iberdomide 0.3 and 0.45 mg at wk 24. Pts were stratified by SLEDAI 2K score (≥10/< 10) and oral corticosteroid (OCS) dose (≥10/< 10 mg/d; max OCS = 20 mg/d prednisone or equivalent). OCS tapering was permitted from wk 8-16. Study duration was 52 wks, and the primary endpoint was SLE Responder Index (SRI-4) response at wk 24. Efficacy analyses were based on the intent-to-treat population that included all pts who were randomized and received ≥1 dose of investigational product. Pts with missing data and treatment failures were imputed as nonresponders.
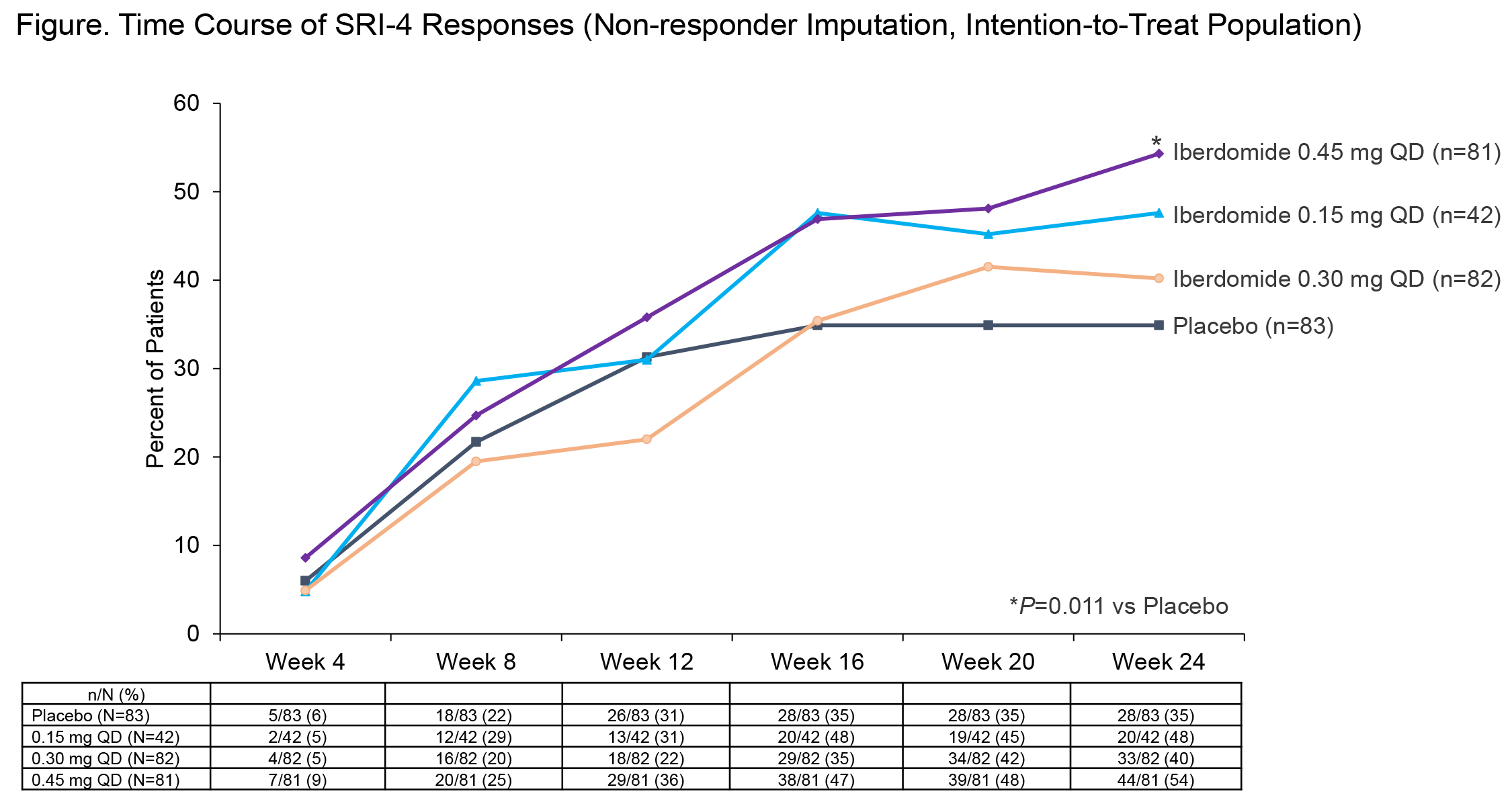
Results: Baseline demographic and disease characteristics were balanced between groups (Table 1). The primary endpoint at wk 24 was met: 54.3% of pts in iberdomide 0.45-mg group achieved SRI-4 response vs 34.9% in PBO group (stratified difference-19.4%; P=0.011). Notably greater SRI-4 responses, vs PBO, were seen starting at wk 16, especially in iberdomide 0.45- and 0.15-mg groups (Figure). Several secondary endpoints were also met. In those with SLEDAI 2K score ≥10, a greater proportion receiving iberdomide 0.45 mg also achieved SRI-4 response at wk 24 (66% vs 39%, nominal P=0.016). SRI-4 response in the prespecified biomarker-defined subset of baseline Aiolos-High was 64% vs 33% (P=0.011) and in pts who were Type 1 IFN-High was 60% vs 33% (P=0.006). At wk 24, iberdomide 0.45 mg was superior to PBO for CLASI-50 response in patients with subacute (92% vs 53%, P=0.035) and chronic cutaneous lupus (62% vs 28%, P=0.029), but not in the overall population. Other endpoints are shown in Table 2. Rates of AEs were 77% vs 65% for iberdomide all doses vs PBO; SAEs (6% vs 8%), severe AEs (4% vs 6%), serious infections (1%, both groups) and AEs leading to discontinuation (8% vs 7%) were comparable. The most common AEs with iberdomide (all doses vs PBO) were urinary tract infection (11%, 4%), upper respiratory tract infection (10%, 5%), neutropenia (8%, 2%), influenza (6%, 4%), nasopharyngitis (5%, 1%), and diarrhea (4%, 0%). Rates of neutropenia and infections were dose-dependent. Neutropenia was mostly grade (gr) 1 and 2 (gr 3, 6%; gr 4, 0.5%) and was reversible, not related to infection risk, and rarely led to discontinuation (2%). Neither systemic opportunistic infections nor tuberculosis occurred. There were 2 thromboembolic events observed with iberdomide and 2 with PBO.
Conclusion:
Iberdomide showed significant efficacy in the treatment of active SLE and was generally well tolerated. Enhanced effects were observed in 2 biomarker-defined populations (Aiolos-High, Type 1 IFN-High), providing a rationale for this novel mechanism in SLE.
To cite this abstract in AMA style:
Merrill J, Werth V, Furie R, van Vollenhoven R, Petronijevic M, Velasco Zamora B, Majdan M, Irazoque-Palazuelos F, Weiswasser M, Korish S, Schafer P, Liu Z, Gaudy A, Agafonova N, Delev N. Efficacy and Safety of Iberdomide in Patients with Active Systemic Lupus Erythematosus: 24-Week Results of a Phase 2, Randomized, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-iberdomide-in-patients-with-active-systemic-lupus-erythematosus-24-week-results-of-a-phase-2-randomized-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-iberdomide-in-patients-with-active-systemic-lupus-erythematosus-24-week-results-of-a-phase-2-randomized-placebo-controlled-study/