Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Filgotinib (FIL) is an oral, potent, selective Janus kinase 1 (JAK1) inhibitor. FINCH 1 (NCT02889796) assessed FIL efficacy and safety in patients (pts) with rheumatoid arthritis (RA) with an inadequate response to methotrexate (MTX-IR); primary outcome results at week (W)12 and W24 were previously reported.1 This analysis presents results from the FINCH 1 study through 52 weeks.
Methods: This global, phase 3, double-blind, active- and placebo (PBO)-controlled study randomized MTX-IR pts with active RA on a background of stable MTX 3:3:2:3 to oral FIL 200 mg or FIL 100 mg once daily, subcutaneous adalimumab (ADA) 40 mg every 2W, or PBO up to W52; pts receiving PBO at W24 were rerandomized to FIL 100 or 200 mg. Efficacy was assessed using clinical, radiographic, and pt-reported outcomes; W52 comparisons were not adjusted for multiplicity, and nominal p-values are reported. Safety endpoints included types and rates of adverse events (AEs) and laboratory abnormalities.
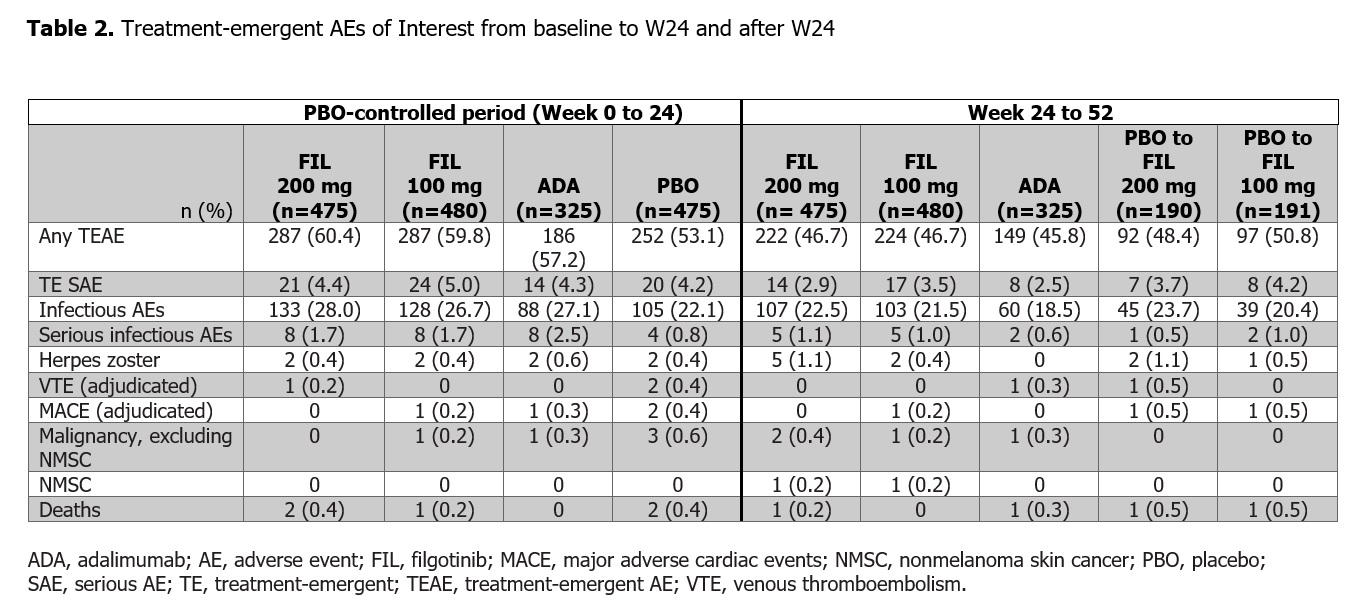
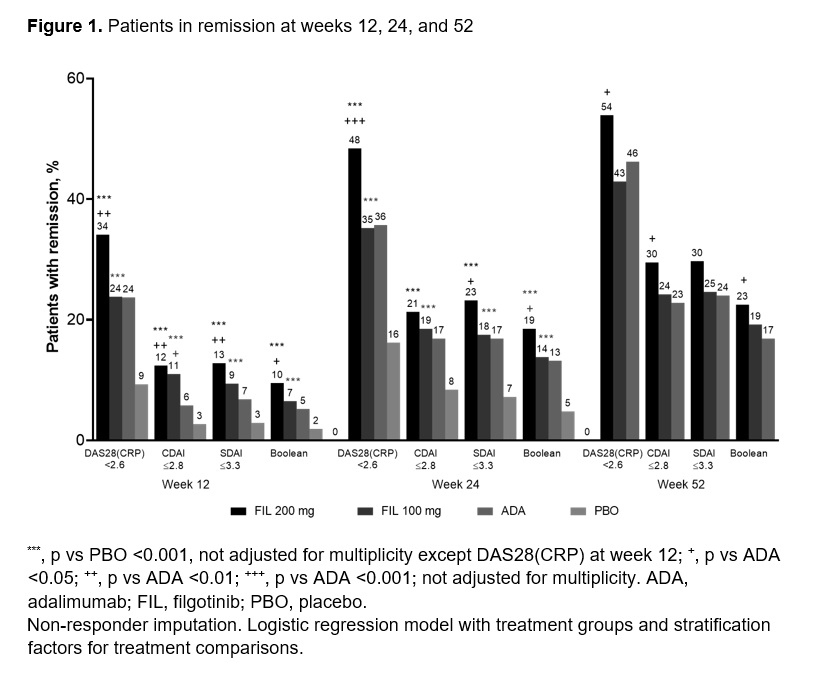
Results: Of 1755 treated pts, 1417 received study drug through W52. The majority (81.8%) were female, mean (standard deviation [SD]) RA duration was 7.8 (7.6) years, and baseline mean (SD) DAS28(CRP) was 5.7 (0.9). FIL efficacy was sustained through W52; 54%, 43%, and 46% of pts receiving FIL 200 and 100 mg and ADA, respectively, had W52 DAS28(CRP) < 2.6 (nominal p for FIL 200 vs ADA = 0.024) (Figure 1, Table 1). FIL safety profile through W52 was consistent with W24 data. AEs of interest were infrequent and balanced among treatments (Table 2).
Conclusion: Through W52, both FIL 200 and 100 mg showed sustained efficacy based on clinical and pt-reported outcomes and radiographic progression and were well tolerated in MTX-IR pts with RA.
- Combe et al., Ann Rheum Dis. 2019; 78 (Suppl 2):77–8.
To cite this abstract in AMA style:
Combe B, Kivitz A, Tanaka Y, van der Heijde D, Simon-Campos J, Baraf H, Kumar U, Matzkies F, Bartok B, Ye L, Guo Y, Tasset C, Sundy J, Jahreis A, Mozaffarian N, Landewé R, Bae S, Keystone E, Nash P. Efficacy and Safety of Filgotinib for Patients with Rheumatoid Arthritis with Inadequate Response to Methotrexate: 52-Week Results [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-filgotinib-for-patients-with-rheumatoid-arthritis-with-inadequate-response-to-methotrexate-52-week-results/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-filgotinib-for-patients-with-rheumatoid-arthritis-with-inadequate-response-to-methotrexate-52-week-results/