Session Information
Session Type: Late-Breaking Abstracts
Session Time: 8:00AM-9:30AM
Background/Purpose: MAS is a life-threatening complication of Still’s disease, characterized by interferon-gamma (IFNg)-driven macrophage activation and systemic hyperinflammation. Emapalumab, an anti-IFNg antibody, binds free and receptor-bound IFNg, providing rapid and targeted neutralization of IFNg. Emapalumab achieved sustained control of MAS in a phase 2 trial (NCT03311854). This report presents data from an expanded population of patients (pts) with MAS in Still’s disease administered emapalumab.
Methods: Data were pooled from two open-label, single-arm interventional studies (NI-0501-06 [NCT03311854] and NI-0501-14 [EMERALD; NCT05001737]) in pts with MAS in Still’s disease who had an inadequate response to high-dose glucocorticoids (GCs). Pts were planned to be treated with emapalumab for 4 weeks: a 6 mg/kg loading dose, followed by 3 mg/kg every 3 days from days 4–16, then 3 mg/kg twice weekly until Day 28 (or longer if insufficient clinical response).1 The primary efficacy endpoint was complete response (CR) at Week 8, defined as resolution of clinical signs according to investigator assessment (visual analog scale [VAS] ≤1/10) and normalization of 7 MAS-related laboratory parameters).1 Overall response (OR) was defined as CR or a partial response. Other endpoints included GC tapering, survival analysis, biomarkers, and safety.
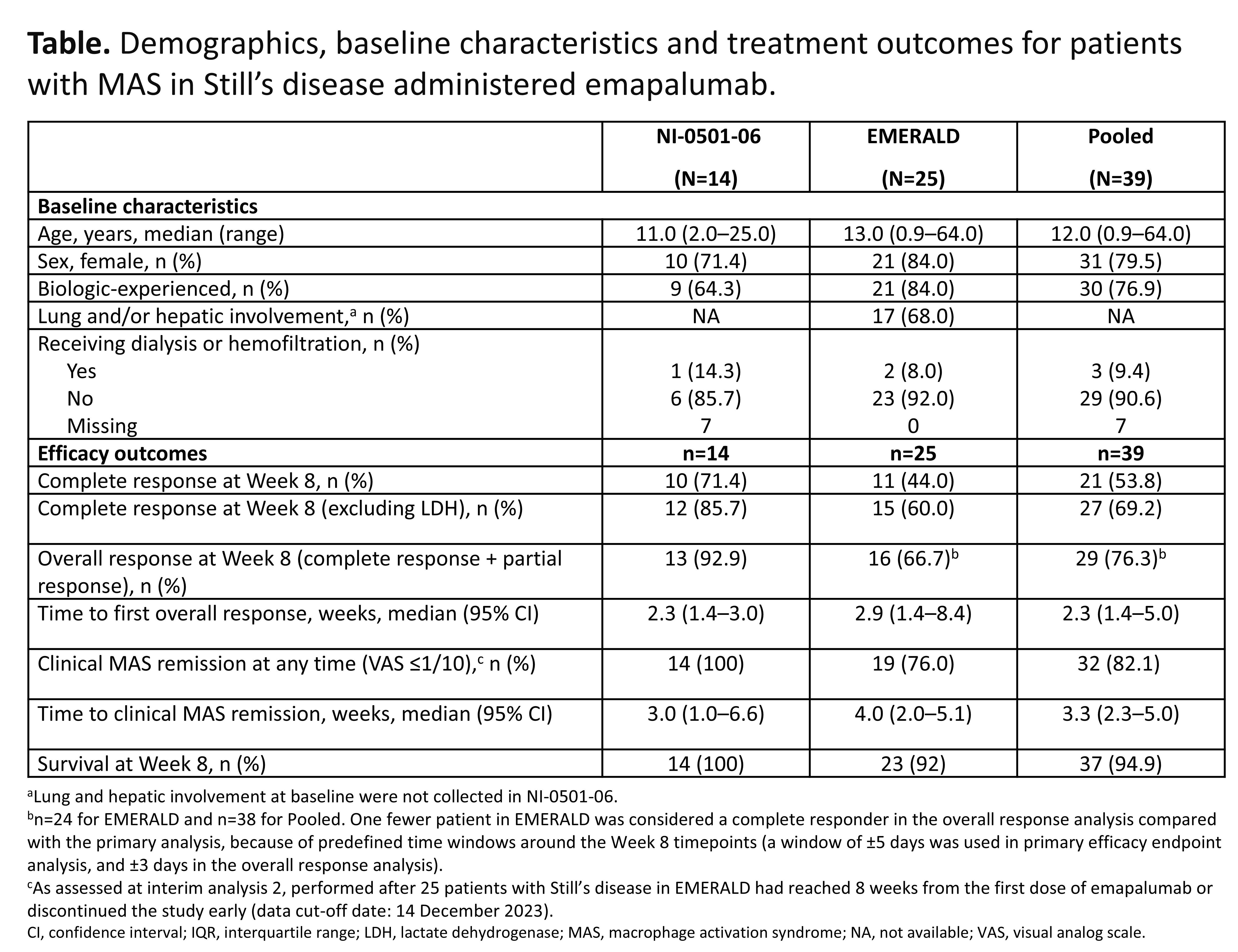
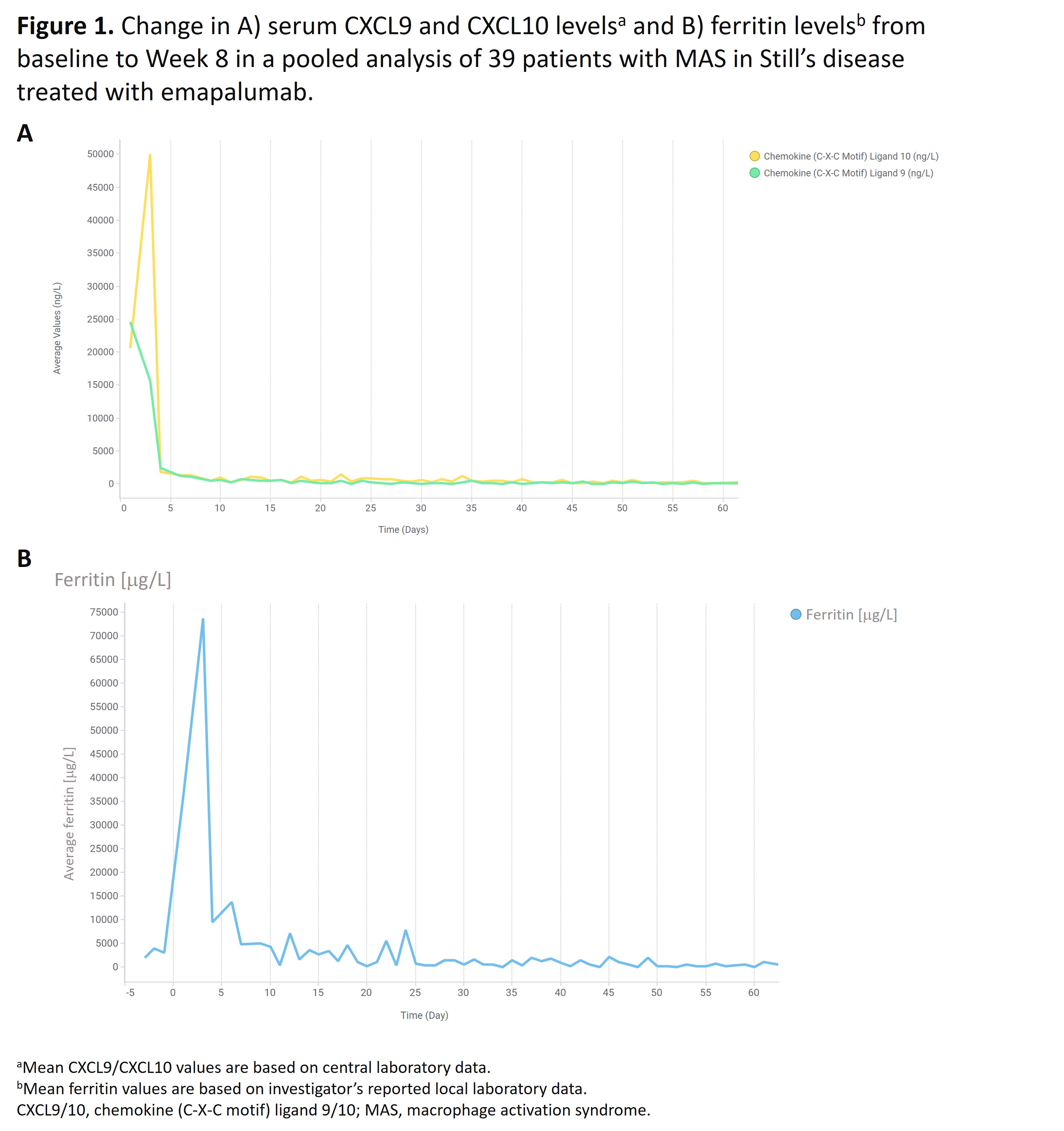
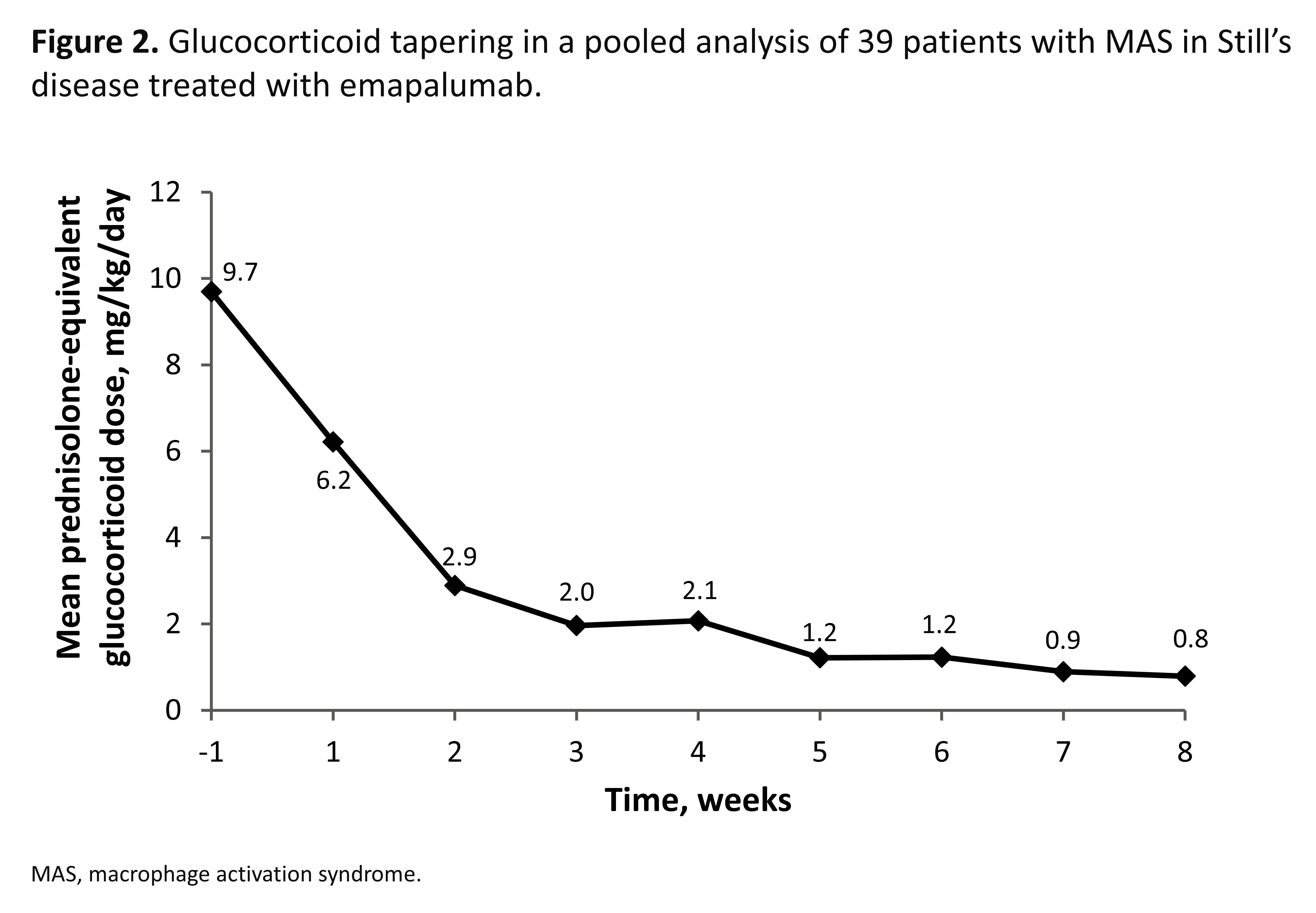
Results: 39 pts with an inadequate response to high-dose GCs were enrolled (31 [79.5%] females), with a median age of 12 years (range 0.9–64), and 30 (76.9%) pts also received biologics for acute MAS in Still’s disease prior to study enrollment (Table). At Week 8, 21 (53.8%) pts achieved a CR (95% confidence interval (CI): 37.2–69.9%) and 33 (85%) pts achieved a CR at any time. The most common CR criteria not met was lactate dehydrogenase (LDH) and, in a post-hoc sensitivity analysis excluding LDH, CR at Week 8 increased to 69.2% (95% CI: 52.4–83.0). 32 (82.4%) pts achieved an OR by Week 8. OR was observed as early as Day 5; median time to first OR was 2.3 weeks. 32 (82.1%) pts achieved investigator-assessed clinical MAS remission at any time (VAS ≤1 cm; median time to clinical remission, 3.3 weeks). 37 (94.9%) pts were alive at Week 8. Biomarkers of hyperinflammation rapidly reduced after initiating treatment with emapalumab (Figure 1). Clinical improvement generally paralleled the degree of IFNg neutralization, assessed using serum chemokine C-X-C ligand 9 (CXCL9), a specific biomarker of IFNg activity. Mean (standard deviation) prednisolone-equivalent GC dose was reduced from 9.7 (9.5) mg/kg/day at Week –1 to 0.8 (0.6) mg/kg/day at Week 8. GCs were tapered to ≤1 mg/kg/day by Week 8 in 28 (72%) pts, and ≤0.5 mg/kg/day in 17 (44%) pts (Figure 2). No new safety concerns were identified. 6 serious adverse drug reactions were reported in 4 pts and 14 infusion-related reactions occurred in 8 pts; none were serious or led to discontinuation of emapalumab infusion.
Conclusion: Emapalumab neutralized IFNg in all pts, as assessed by CXCL9, rapidly controlled MAS in >80% of pts and supported clinically meaningful GC dose reduction in 72% of pts with MAS in Still’s disease with an initial inadequate response to high-dose GC treatment.
References
1. De Benedetti F, et al. Ann Rheum Dis 2023;82:857–865.
To cite this abstract in AMA style:
Grom A, Ullman U, Mahmood A, Blomkvist J, Jamieson B, De Benedetti F. Efficacy and Safety of Emapalumab in Children and Adults with Macrophage Activation Syndrome (MAS) in Still’s Disease: Results from a Phase 3 Study and a Pooled Analysis of Two Prospective Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-emapalumab-in-children-and-adults-with-macrophage-activation-syndrome-mas-in-stills-disease-results-from-a-phase-3-study-and-a-pooled-analysis-of-two-prospective-tri/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-emapalumab-in-children-and-adults-with-macrophage-activation-syndrome-mas-in-stills-disease-results-from-a-phase-3-study-and-a-pooled-analysis-of-two-prospective-tri/