Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: RA – Treatment I: Switching or Discontinuation of Therapies
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Curcumin, extracted from Curcuma (Turmeric) spices, has been shown to have anti-inflammatory properties in animal models of arthritis and in observational studies(1,2). Despite the lack of well controlled studies, it is widely used as a dietary supplement by patients with Rheumatoid Arthritis (RA) across the world. DMARD-free remission is maintained in less than one-fourth of patients with RA.
We explored the potential of Curcumin in maintaining remission in patients with RA while tapering conventional synthetic DMARDs (csDMARDS).
Methods: In this single centre, patient and investigator blinded trial, adults with RA in sustained remission [DAS-28 < 2.6 and SDAI ≤ 3.3 and TJC (44) < 0 and SJC (44) < 0 confirmed during two consecutive visits] for more than 6 months were randomised in a 1:1 ratio to receive standardized Curcumin- piperine preparation (1 gm Curcumin with 5 mg Piperine twice daily) or matching placebo . cs-DMARDS were tapered and stopped sequentially during each visit as per a fixed protocol in the following sequence: Methotrexate >Leflunomide > Sulfasalazine > Hydroxychloroquine. Patients who had required biological DMARDs, corticosteroids or NSAIDs in last 6 months or those already on curcumin supplements had been excluded before randomization.
The primary outcome was flare-free survival at the end of 52 weeks (per protocol analysis). Standard definitions were used to define flares. Patients with flares received rescue therapy and were excluded from further follow-up. Secondary outcome measures were time to flare, safety of curcumin, the estimation of serum curcuminoid levels and its correlation with flares.(Trial registration no: CTRI/2018/04/013279)
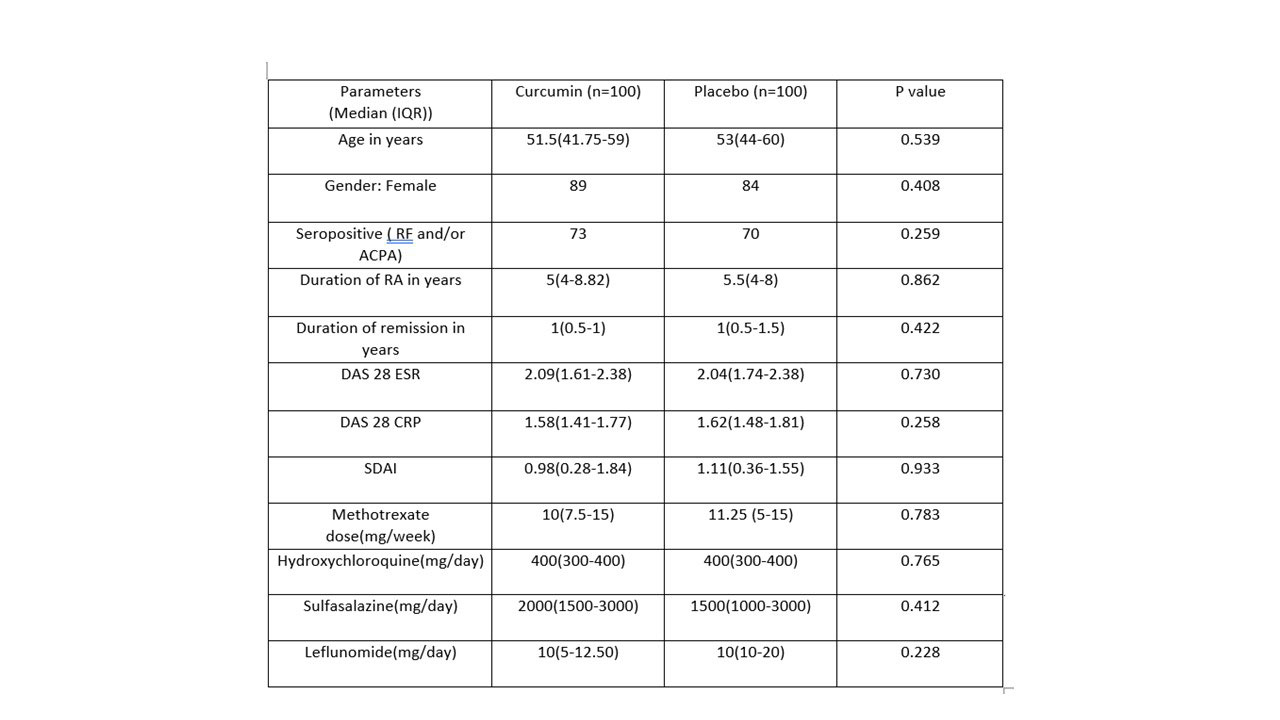
Results: After screening 274 patients with RA, 200 were randomized 1:1 to Curcumin or to placebo groups. Baseline characteristics were comparable between the groups [Table 1]. Per-protocol analysis included 92 participants in the curcumin group and 93 in the placebo group. At 52 weeks follow-up, flare-free survival was same between both groups [Figure 1; Log-rank test: p=0.76]. The median time to flare in both groups was statistically same [Curcumin group: 219 days (IQR: 123) versus 214 days (95.8); p=0.067].
Adverse effects were similar across both groups with one each of infection and benign tumour in the curcumin group; and 2 new diagnoses of psoriasis in the placebo group.
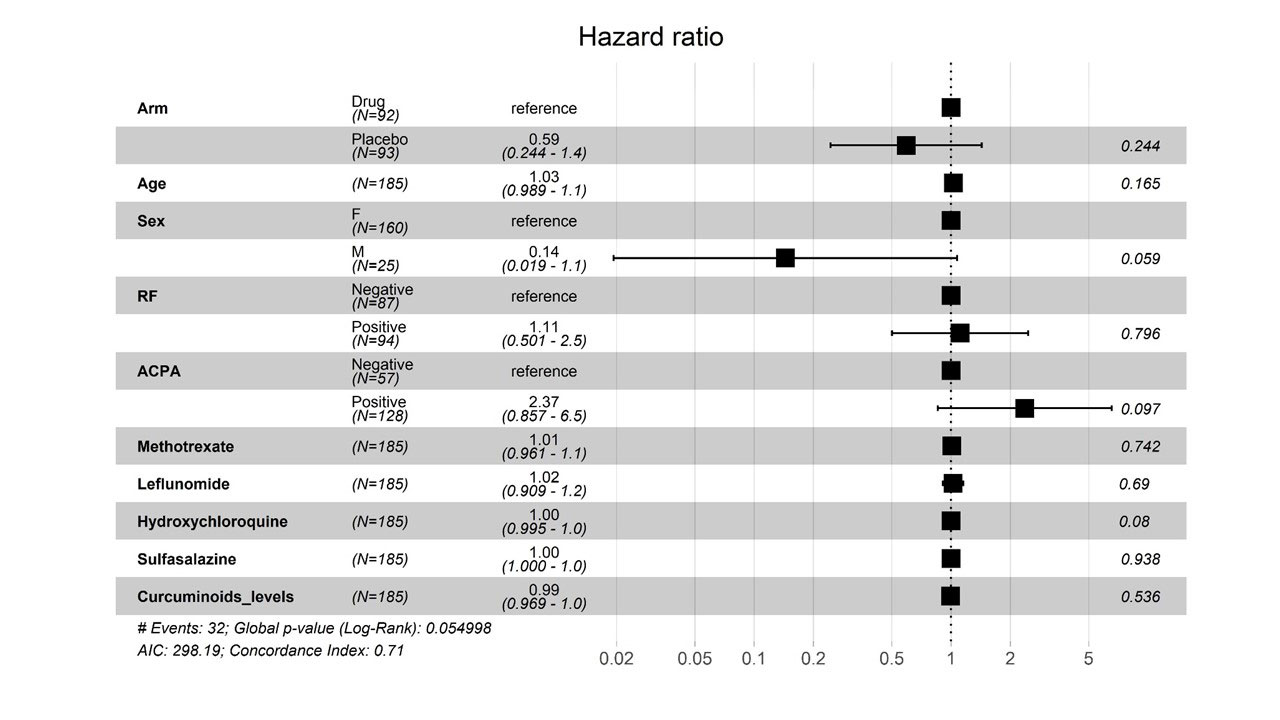
The curcuminoid levels , estimated in 72/92 in the Curcumin group and 70/93 in the placebo group showed a significantly higher serum level of curcuminoids in the interventional arm [ Median (IQR): 19.70 ng/ml (31-12) vs 0.00(0-3.0), P =< 0.01], confirming the good bioavailability of the preparation used. Cox proportionate regression modelling showed that flares were independent of serum curcuminoid levels [Adjusted HR=0.99(95%CI: 0.969-1.0); p=0.5]. The model also showed the lack of association of flare-free survival with age, sex, seropositivity, or DMARDs used at baseline [Figure 2].
Conclusion: In RA patients who are in remission in whom csDMARDs were being systematically tapered, the addition of Curcumin, despite having a good bioavailability, did not affect the flare-free survival or time to flare.
To cite this abstract in AMA style:
Shenoy P, Bhatt S, Reji R, Vijayan A, Mohanan M, Ahmed S, Mehta P, Paul A. Efficacy and Safety of Curcumin in Maintaining Remission During Disease Modifying Anti Rheumatic Drug Withdrawal in Rheumatoid Arthritis at 52 Weeks: Phase III Double-Blind Randomized Placebo Controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-curcumin-in-maintaining-remission-during-disease-modifying-anti-rheumatic-drug-withdrawal-in-rheumatoid-arthritis-at-52-weeks-phase-iii-double-blind-randomized-placebo-controll/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-curcumin-in-maintaining-remission-during-disease-modifying-anti-rheumatic-drug-withdrawal-in-rheumatoid-arthritis-at-52-weeks-phase-iii-double-blind-randomized-placebo-controll/