Session Information
Date: Monday, November 6, 2017
Title: Osteoarthritis – Clinical Aspects Poster I: Clinical Trials and Interventions
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: CNTX-4975, a highly purified, synthetic trans-capsaicin, targets transient receptor potential vanilloid 1, producing analgesia via reversible desensitization of end terminals of primary afferent pain fibers within the joint. This phase 2 dose-ranging study evaluated CNTX-4975 in subjects with osteoarthritis (OA) knee pain.
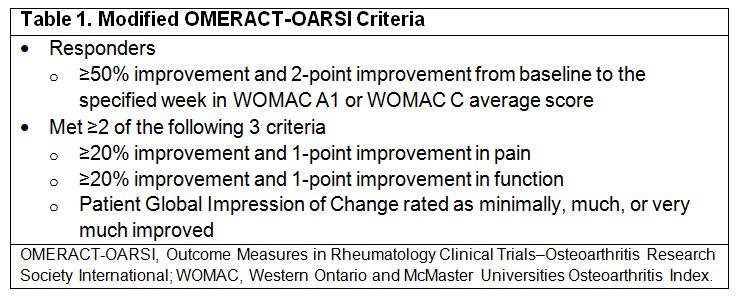
Methods: Subjects aged 45–80 years with chronic knee OA and stable moderate to severe knee pain who failed previous oral/intra-articular (IA) analgesics were randomized 2:1:2 to a single IA injection of placebo, CNTX-4975 0.5 mg, or CNTX-4975 1.0 mg. Randomization was stratified by Kellgren-Lawrence (K-L) grade (2–3 vs 4) and body mass index (<30 vs ≥30 kg/m2). The primary efficacy endpoint was area under the curve (AUC) for change from baseline in daily Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) A1 score (pain with walking) through week 12 (analysis of covariance). Additional efficacy endpoints included mean change from baseline in weekly WOMAC A1 score, WOMAC B stiffness subscale, and WOMAC C physical function subscale through week 24 (mixed model for repeated measures). Symptomatic improvements were assessed with modified Outcome Measures in Rheumatology Clinical Trials–Osteoarthritis Research Society International (OMERACT-OARSI) criteria (Table 1). Statistical tests were 2-sided (alpha, 0.10). Safety assessments included treatment-emergent adverse events (TEAEs).
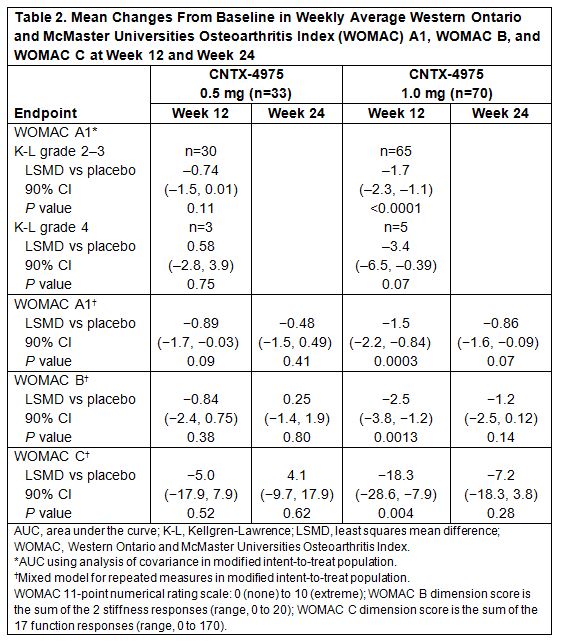
Results: Efficacy was evaluated in 172 subjects (placebo, n=69; CNTX-4975 0.5 mg, n=33; CNTX-4975 1.0 mg, n=70), most with K-L grade 2–3 (placebo, n=62; CNTX-4975 0.5 mg, n=30; CNTX-4975 1.0 mg, n=65). Mean baseline WOMAC A1 pain score was 7.3 (Numeric Pain Rating Scale, 0–10). WOMAC A1 scores (AUC analysis) were significantly improved vs placebo at week 12 with CNTX-4975 0.5 mg (least squares mean difference [LSMD]: −0.8; P=0.07) and 1.0 mg (LSMD: −1.6; P<0.0001) and at week 24 with CNTX-4975 1.0 mg (LSMD: –1.4; P=0.0002). Additional efficacy analyses are reported in Table 2. At week 12, more subjects treated with CNTX-4975 0.5 mg and 1.0 mg met modified criteria for OMERACT-OARSI response (76% [P=0.11] and 86% [P=0.0005], respectively) vs placebo (59%). The incidence of TEAEs was 30% for placebo or CNTX-4975 1.0 mg and 47% for CNTX-4975 0.5 mg at week 24. Most TEAEs were considered unrelated to study treatment. Arthralgia was the most common TEAE with placebo and CNTX-4975 1.0 mg.
Conclusion: A single IA injection of CNTX-4975 1.0 mg improved pain with walking, knee stiffness, and physical function and was well tolerated in subjects with moderate to severe OA knee pain.
To cite this abstract in AMA style:
Stevens R, Petersen D, Ervin J, Nezzer J, Nieves Y, Campbell J, Guedes K, Burges R, Hanson P. Efficacy and Safety of Cntx-4975 in Subjects with Moderate to Severe Osteoarthritis Knee Pain: 24-Week, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-cntx-4975-in-subjects-with-moderate-to-severe-osteoarthritis-knee-pain-24-week-randomized-double-blind-placebo-controlled-dose-ranging-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-cntx-4975-in-subjects-with-moderate-to-severe-osteoarthritis-knee-pain-24-week-randomized-double-blind-placebo-controlled-dose-ranging-study/