Session Information
Session Type: ACR Late-breaking Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose:
Atacicept targets B-cell stimulating factors BLyS and APRIL. In the APRIL-SLE trial, atacicept 150 mg was associated with reduced SLE flares in post-hoc analyses.1Methods:
ADDRESS II was a phase IIb, multicenter study (NCT01972568). Patients with active (SLEDAI-2K ≥ 6), autoantibody-positive SLE on standard-of-care therapy were randomized (1:1:1) to weekly SC injections of atacicept (75 or 150 mg) or placebo (PBO) for 24 weeks. The primary endpoint was the % of patients achieving SRI-4 response at week 24.Results:
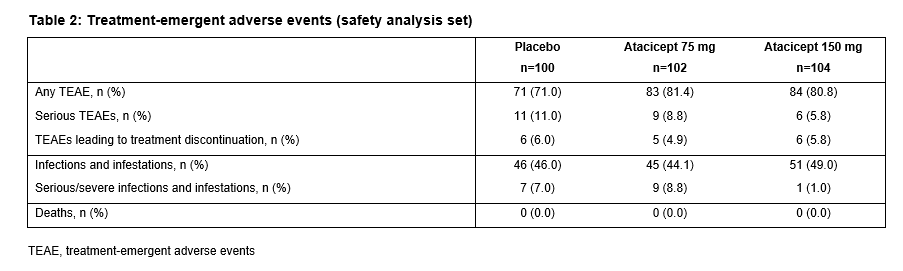
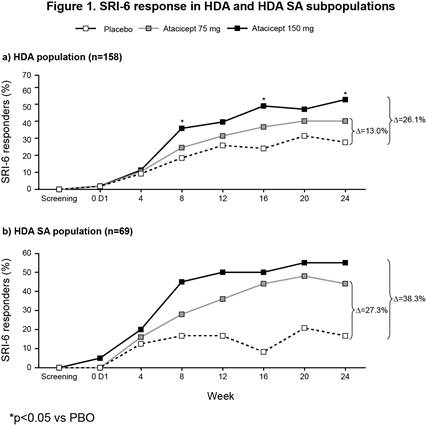
The ITT population included 306 patients (100 PBO; 102 atacicept 75 mg; 104 atacicept 150 mg). There was a trend towards improved SRI-4 response with atacicept vs PBO at week 24 (p=ns) (primary analysis; screening visit as baseline, BL) (Table 1). In a prespecified sensitivity analysis using study day 1 as BL, a significantly larger proportion of patients on atacicept achieved SRI-4 response at week 24: PBO, 41%; atacicept 75 mg, 55.9% (OR 1.88) p=0.029; atacicept 150 mg, 55.8% (OR 1.96) p=0.020. In predefined subpopulations with high disease activity (HDA; SLEDAI-2K ≥10, n=158), serologically active (SA) disease (dsDNA antibody-positive and low complement, n=84), or both (HDA SA, n=69), enhanced improvements in SRI-4 and SRI-6 response rates were seen with atacicept vs PBO (Table 1). SRI-6 response rates in HDA patients were significantly greater with atacicept 150 mg vs PBO at weeks 8, 16 and 24 (Figure 1). In HDA patients, severe flare was significantly reduced by atacicept 75 mg (BILAG A HR 0.1; p=0.002; SLEDAI flare index [SFI] HR 0.3; p=0.029) and 150 mg (BILAG A HR 0.3; p=0.038; SFI HR 0.2; p=0.004). In the ITT population, BILAG A flare was significantly reduced vs PBO with atacicept 75 mg (p=0.019), and severe SFI flare reduced with 150 mg (p=0.002). Atacicept was associated with increased serum complement C3/C4, and decreased IgG, IgM, IgA, and anti-dsDNA antibodies over time. Rates of AEs were similar between groups. Compared with placebo, the risks of SAEs and serious/severe infections were not increased with atacicept (Table 2).Conclusion:
Atacicept showed evidence of efficacy in SLE, particularly in HDA and SA patients; reduction in disease activity and severe flare was observed. Atacicept was associated with a favorable safety profile. 1. Isenberg D, et al. Ann Rheum Dis. 2015;74:2006-15.
Disclosure: J. T. Merrill, Anthera Pharmaceuticals, Lilly, 5; D. J. Wallace, Merck Serono, 5; S. Wax, EMD Serono, 3; A. Kao, EMD Serono, 3; P. Fraser, EMD Serono, 1,EMD Serono, 3; W. Chin, EMD Serono, 3; D. A. Isenberg, Merck Serono, 5.
To cite this abstract in AMA style:
Merrill JT, Wallace DJ, Wax S, Kao A, Fraser P, Chin W, Isenberg DA. Efficacy and Safety of Atacicept in Patients with Systemic Lupus Erythematosus: Results of a 24-Week Randomized, Placebo-Controlled, Phase IIb Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-atacicept-in-patients-with-systemic-lupus-erythematosus-results-of-a-24-week-randomized-placebo-controlled-phase-iib-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-atacicept-in-patients-with-systemic-lupus-erythematosus-results-of-a-24-week-randomized-placebo-controlled-phase-iib-study/