Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Adrenocorticotropic hormone
(ACTH) gel (repository corticotropin injection) is a
long-acting full sequence ACTH that may include other pro-opiomelanocortin
peptides thought to have anti-inflammatory and immunomodulatory
effects through melanocortin receptors. Its approval by
FDA for polymyositis (PM) and dermatomyositis
(DM) in 1952 was based on few case reports. We sought to evaluate the efficacy,
safety, tolerability and steroid-sparing effect of ACTH gel in refractory adult
PM and DM patients in a 6 month prospective, open-label uncontrolled pilot
trial.
Methods: 12 adult patients (5 PM; 7 DM)
were enrolled at 2 centers. ACTH gel was given as 80 U twice weekly by self-injection.
One DM patient withdrew consent before study drug. The primary outcome was the proportion
of patients meeting definition of
improvement (DOI), defined by IMACS as improvement of ≥ 20% in 3 of 6
core set measures (CSM) with no more than 2 worsening by ≥25% [(which cannot
include the manual muscle testing (MMT)]. CSM include MD global, patient
global, MMT, health assessment questionnaire (HAQ), muscle enzymes and
extra-muscular global assessment. Secondary endpoints included steroid-sparing
effect, safety, tolerability and recently proposed myositis response criteria.
Results: Eleven patients (5 PM; 6 DM)
were analyzed. Median age was 51 (IQR 37.9, 58.7), with 91% females and 46%
Caucasians. All patients “failed” prednisone and a median (IQR) of 2 (2-3)
additional immunosuppressive agents. Although the trial is ongoing, 8 patients completed
6 months on the drug and 3 have completed 1, 2 and 4 months in the trial,
respectively. One patient stopping drug due to heart block at 2 months is considered
a treatment failure. 91% (10/11) of subjects met the primary outcome by a median
(IQR) of 3 (2-4) months, but the response was not sustained in 2 patients (on
drug). Sustained improvement (DOI at subsequent visits) was seen in 8 (73%)
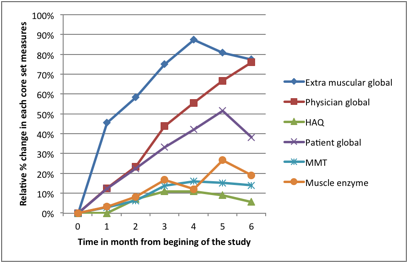
patients. Median relative % improvement in MD global was 73%, 38% in patient
global, 14% in MMT, 78% in extra muscular global, 13% in HAQ, 7% in muscle enzymes (Figure 1).
Regarding the new myositis response criteria, 9 patients achieved minimal, 6
moderate and 4 major improvement with a median (IQR) total improvement score of
40 (25-65) on scale of 0-100. ACTH gel was safe, well-tolerated, and steroid-sparing
with a drop in the median (IQR) prednisone dose from 15 mg (7.5-30) at baseline
to 1.25 mg (0-4) at last visit (p=0.001). There were 4 serious adverse events
in 3 patients: 2 with herpes zoster related to drug and 1 with musculoskeletal
chest pain and 1 with heart block, both unrelated to study drug.
<>Conclusion: ACTH gel
improved most enrolled myositis patients and was steroid sparing, safe and well
tolerated. Viral infections require monitoring. A randomized controlled trial should
be considered to further assess its efficacy in myositis.
Figure 1: Median relative
percent change in core set measures on treatment with ACTH gel.
To cite this abstract in AMA style:
Aggarwal R, Marder G, Loganathan P, Koontz D, Nandkumar P, Qi Z, Oddis CV. Efficacy and Safety of Adrenocorticotropic Hormone Gel (Acthar Gel ®) in Refractory Dermatomyositis or Polymyositis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-adrenocorticotropic-hormone-gel-acthar-gel-in-refractory-dermatomyositis-or-polymyositis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-adrenocorticotropic-hormone-gel-acthar-gel-in-refractory-dermatomyositis-or-polymyositis/