Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Safe and effective therapies are needed for OA pain. MM-II, a novel suspension of large, empty, multilamellar liposomes composed of dimyristoylphosphatidylcholine and dipalmitoylphosphatidylcholine, effectively reduced cartilage degeneration in OA animal models administered weekly IA injections. A single IA injection of MM-II lowered knee pain in OA patients for up to 3 months in a first-in-human study. This phase 2b study evaluated dosing, safety, and efficacy of MM-II through 26 weeks in patients with symptomatic knee OA.
Methods: Consenting patients were enrolled in a 6-arm, randomized, double-blind, placebo (PBO)-controlled, 26-week trial evaluating one IA injection of 1, 3, and 6 mL of MM-II (150 mM lipids) and corresponding volumes of PBO. Key inclusion criteria were age ≥40 years, radiographic Kellgren-Lawrence grades 2 or 3 in the index knee, ACR criteria for OA, WOMAC A pain level ≥2/4 within 24 hours of baseline (BL), index knee visual analog scale pain score of 50 to 90 mm for ≥5/7 days prior to BL, and intolerance or inadequate response to NSAIDs or acetaminophen. Patients with moderate to large effusions in the index knee or with moderate to severe pain in another joint were excluded. The primary endpoint was change in WOMAC A pain score at week 12; secondary endpoints included change from BL in WOMAC A pain, weekly average daily knee pain score, and use of rescue medication. Randomization was stratified by BMI and BL index knee pain. The primary endpoint was analyzed for all patients who received MM-II or PBO (full analysis set) using mixed models for repeated measures. Treatment differences were estimated with least square means (LSM), and multiplicity-adjusted P-values were calculated for MM-II vs PBO 3 mL WOMAC A scores using step-down Dunnett’s hierarchical testing. Nominal P-values were not adjusted for multiplicity.
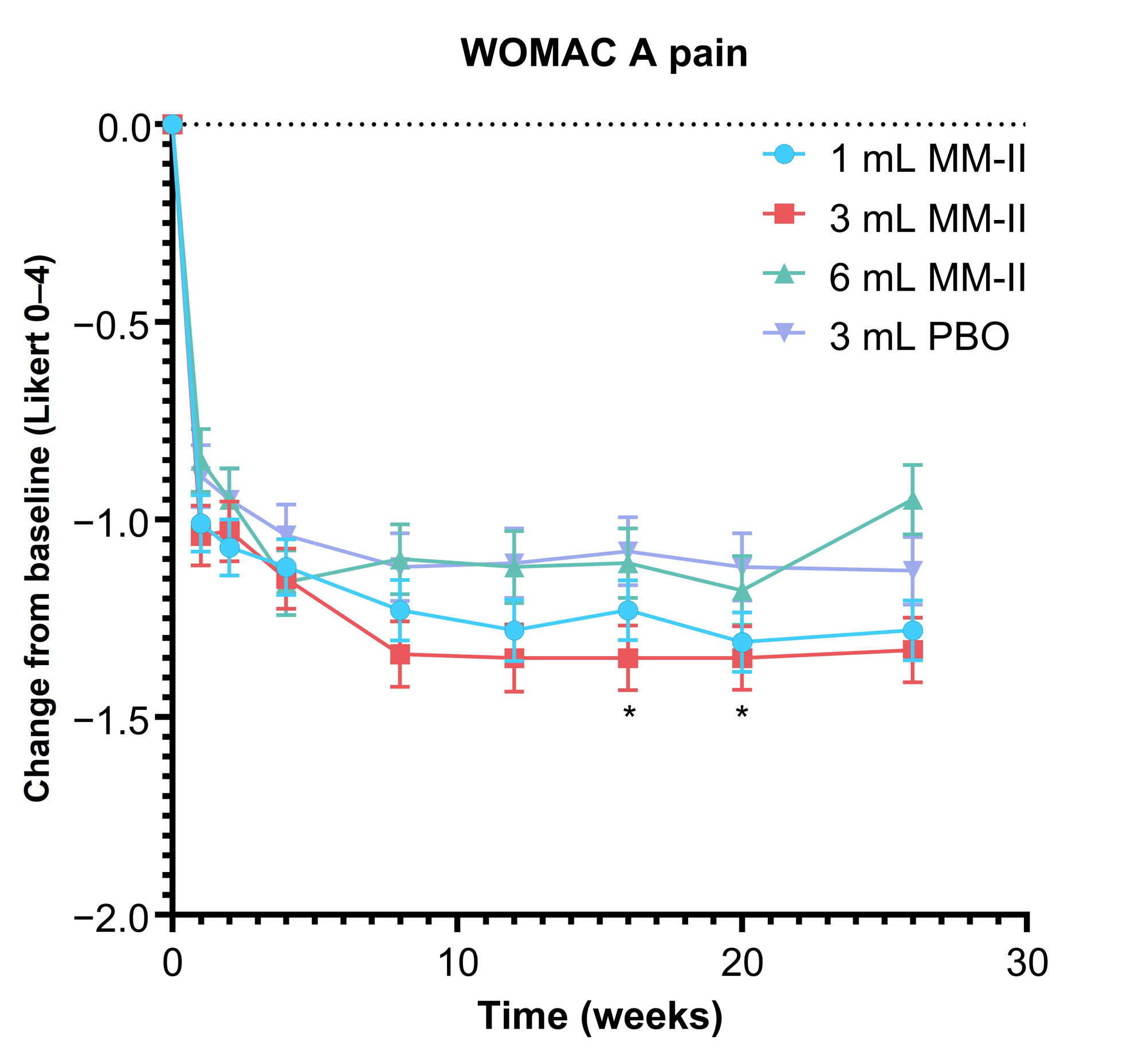
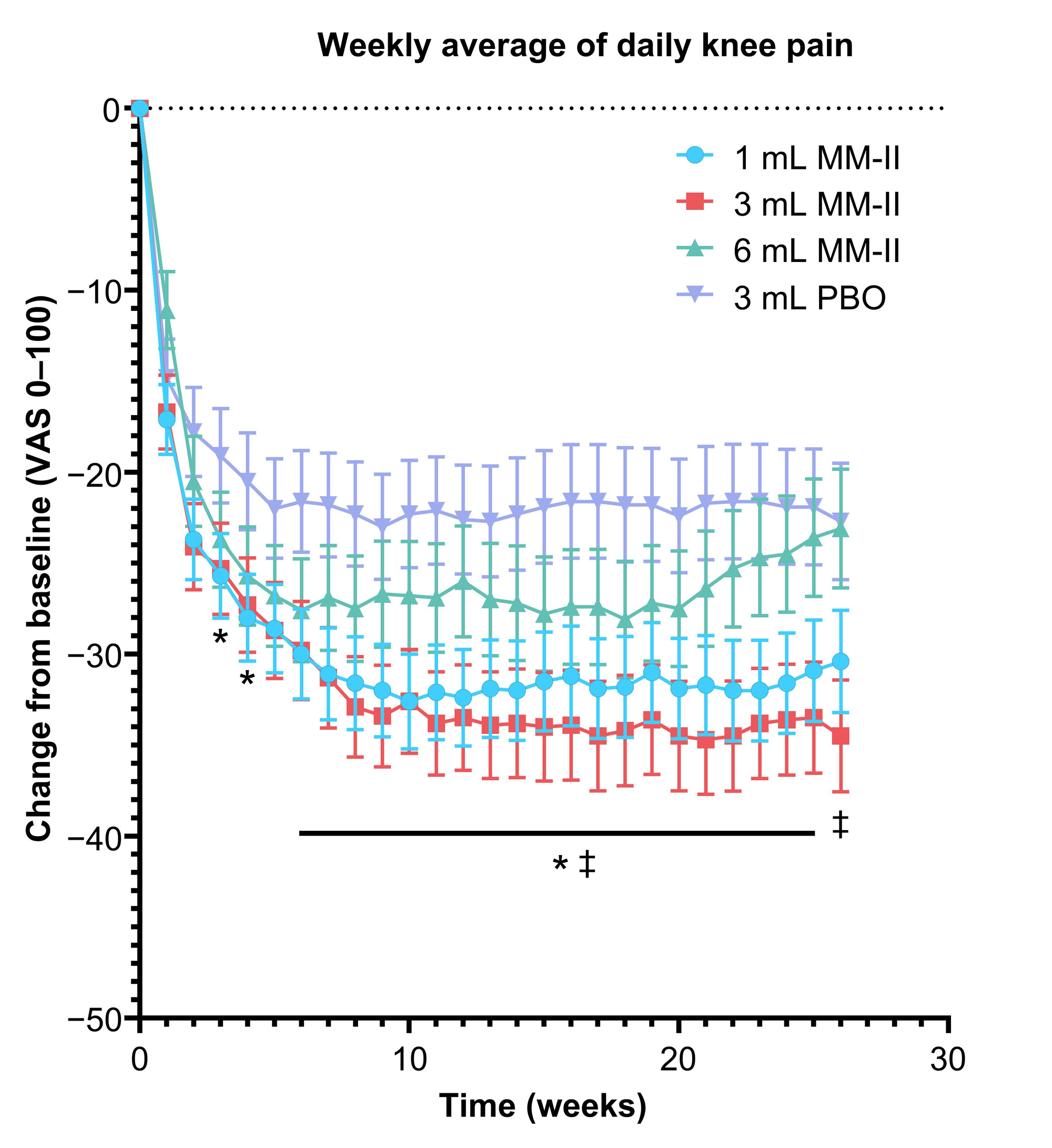
Results: Of 397 enrolled patients, 258 (65.0%) were female and 266 (67.0%) were White. Mean age and BMI were 62.7 years (standard deviation [SD], 8.1) and 30.8 kg/m2 (SD, 6.1), respectively. Treatment groups did not significantly differ at BL. Overall, 7.1% of patients discontinued the study. At week 12, LSM change from BL WOMAC A pain was −0.24 for the MM-II 3 mL group (95% confidence interval [CI], −0.476 to −0.004; P = 0.085), −0.16 for the 1 mL group (95% CI, −0.390 to 0.061; P = 0.850), and −0.02 for the MM-II 6 mL group (95% CI, −0.269 to 0.222; P = 0.850). In the 3 mL group, results were sustained until week 26 with nominal significance at 2 time points (Figure 1). When comparing MM-II 3 mL to the pooled PBO groups, LSM difference in change from BL of WOMAC A pain at week 12 was −0.28 (95% CI, −0.484 to −0.068; P = 0.018). LSM differences in weekly average daily pain scores for the MM-II 3 mL group were nominally significant from week 6 through 26 (nominal P < 0.05 for all; Figure 2). Changes in rescue medication use reflected changes in symptoms. Treatment-emergent serious adverse events (AEs) were reported in 2.67% of MM-II and 2.99% of PBO patients; incidence of injection site AEs were 1.91% and 2.99%, respectively.
Conclusion: MM-II treatment was well tolerated and may provide durable, clinically relevant pain relief. These data support phase 3 studies.
PBO, placebo.
PBO, placebo; VAS, visual analog scale.
To cite this abstract in AMA style:
Thomas S, Rovsing H, Lau E, Boll S, Brahmachari B, Chou R, Joshi T, Wechsler R, Yao S, Weiner S, Bihlet A, Conaghan P. Efficacy and Safety of a Single Intra-Articular Injection of MM-II, a Novel Suspension of Large, Empty, Multilamellar Liposomes, in People with Painful Knee OA: Results of a 26-Week, Phase 2b, Placebo-Controlled, Double-Blind, Randomized Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-a-single-intra-articular-injection-of-mm-ii-a-novel-suspension-of-large-empty-multilamellar-liposomes-in-people-with-painful-knee-oa-results-of-a-26-week-phase-2b-placebo/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-a-single-intra-articular-injection-of-mm-ii-a-novel-suspension-of-large-empty-multilamellar-liposomes-in-people-with-painful-knee-oa-results-of-a-26-week-phase-2b-placebo/