Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: A biosimilar is a medication that is highly similar to a biologic agent that is already approved by a regulatory organization. Considering the share of health care expenditures attributable to biologic medicines, the use of a biosimilar presents a chance to reduce costs. Rituximab is an effective treatment option for maintaining remission in ANCA-associated vasculitis (AAV). Our goal was to compare the efficacy and safety of the rituximab biosimilar Ruxience® [Rituximab-pvvr (RTX-pvvr)], to that of its originator, Rituxan® [Rituximab (RTX)], in maintaining remission in patients with granulomatosis with polyangiitis (GPA).
Methods: This is a retrospective, single-center cohort study based on data obtained from electronic medical records. Eligible patients were adults with GPA, followed at the Vasculitis Center for at least two outpatient visits who were in remission (BVAS/WG≤ 1 and Prednisone dose≤ 10 mg/day) after receiving RTX for at least one year of maintenance therapy and then transitioned to an equivalent dose of RTX-pvvr for continuation of maintenance therapy in 2021. The primary endpoint was remission rate (BVAS/WG ≤ 1 and/or Prednisone dose ≤ 10 mg/day) at 6 months after transitioning to RTX-pvvr. Secondary endpoints were: 1- Infections requiring hospitalization; 2- Infusion reactions (classified based on National Cancer Institute Common Terminology Criteria for Adverse Events), and 3- Patient reported outcomes measurement (PROMIS) among RTX vs RTX-pvvr. McNemar test was performed for assessment of primary and secondary endpoints. Categorical Measures were summarized with frequency (%), and analyzed with Pearson’s Chi-square or Fisher’s exact test, where appropriate. Continuous measures were summarized with mean (SD) or median (25th, 75th quartile), and analyzed with two-sample t-tests, or Wilcoxon-Mann Whitney tests depending on the normality of the data.
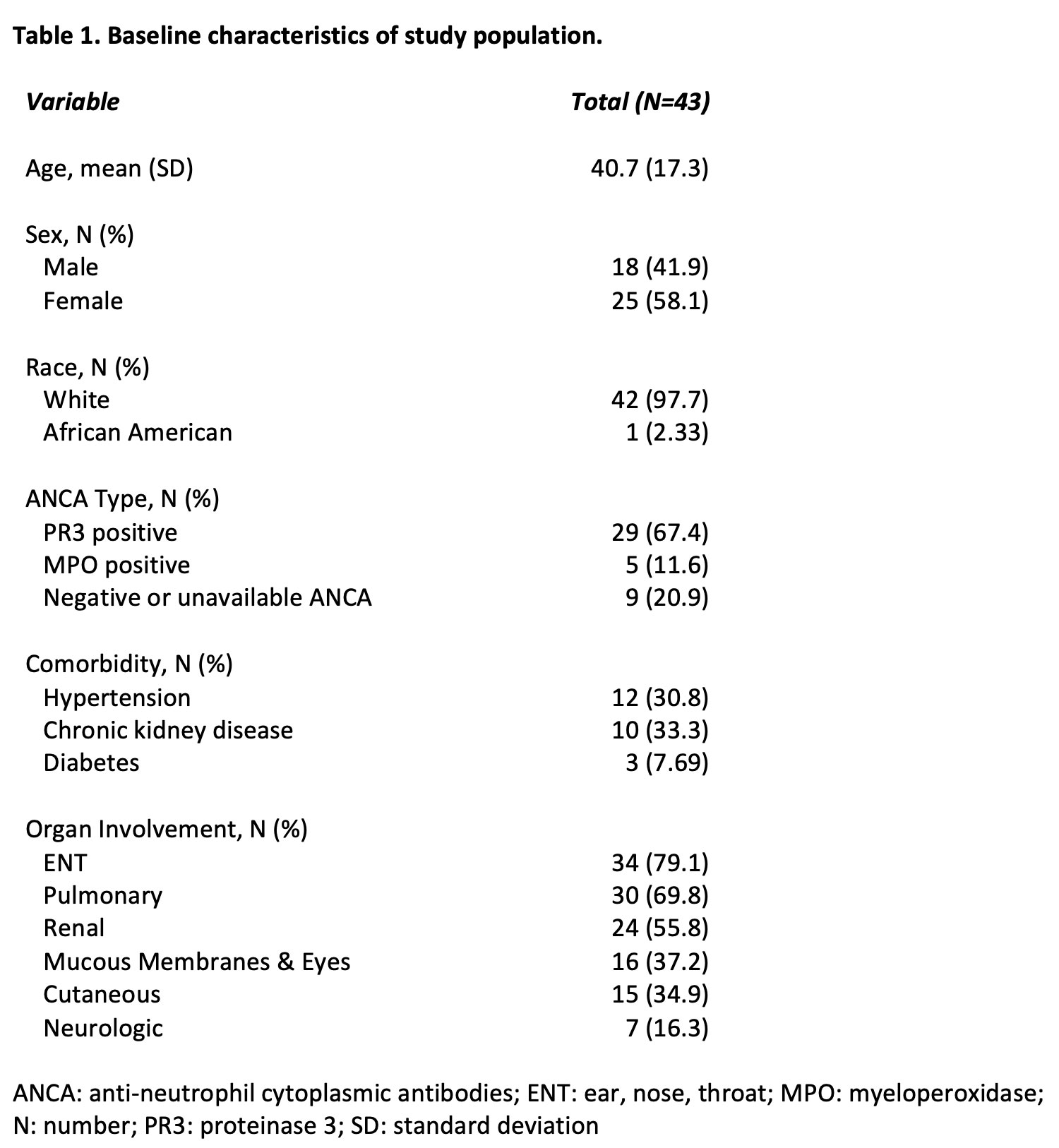
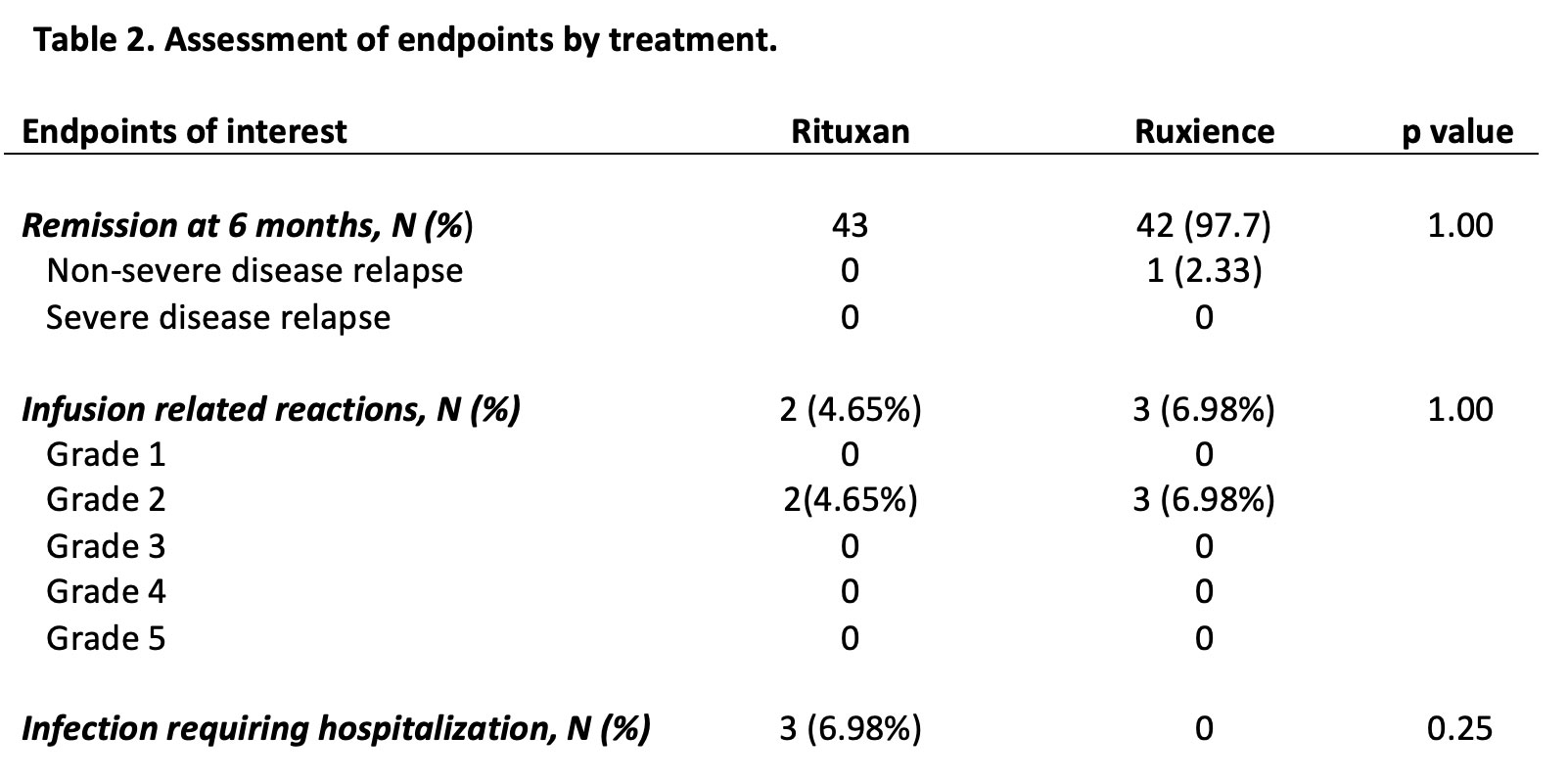
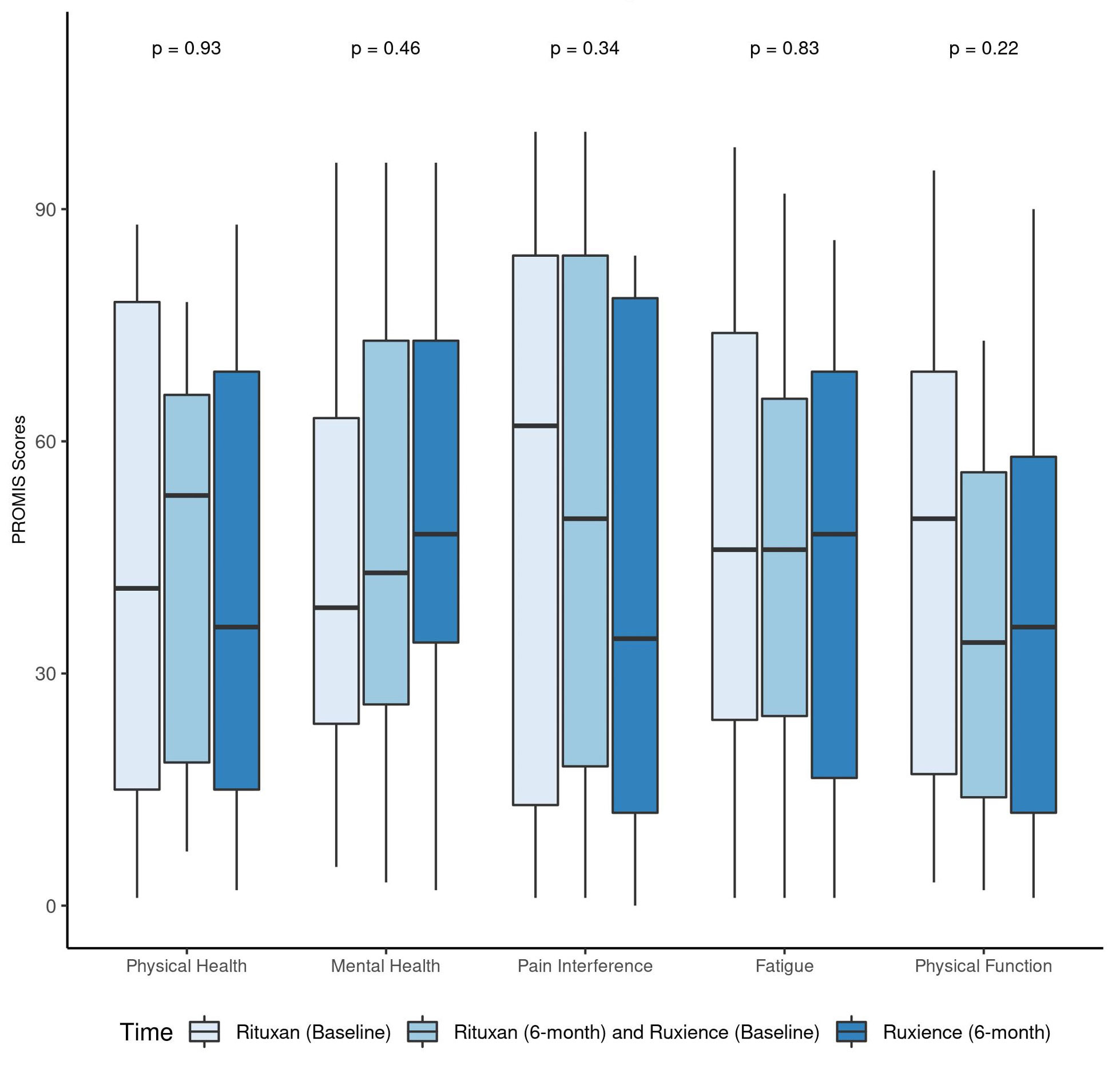
Results: A total of 43 patients met the inclusion criteria. 58.1% were female, 97.7% white, with a mean age of 40.7 years (SD 13.7)(Table-1). The most commonly involved organ systems were ENT (79.1%), pulmonary (69.8%) and renal (55.8%). Of the 43 patients in remission who transitioned to RTX-pvvr, 42 (97.7%) remained in remission, while 1 (2.3%) had a non-severe disease relapse (BVAS/WG=2) and none had a severe disease relapse (p=1.0) (Table-2). Three out of 43 (6.98%) patients on RTX had infections requiring admission for intravenous antibiotics throughout the 6-month post-RTX infusion, whereas no infections were reported on RTX-pvvr (p=0.248). Infusion related reactions were reported in 2 (4.65%) and 3 (6.98%) out of 43 patients on RTX and RTX-pvvr (p=1.0), respectively. All infusion reactions recorded were grade 2 infusion reactions requiring infusion interruption briefly and symptomatic treatment. PROMIS data did not show any differences between the 2 cohorts (Figure-1).
Conclusion: Our results suggest that stable patients on maintenance RTX therapy remained in remission when switched to RTX-pvvr in the majority of patients with GPA. Infusion reactions, infection rates and PROMIS data were comparable among patients who received RTX-pvvr and RTX. Larger studies with larger number of patients are needed to validate our results.
To cite this abstract in AMA style:
Vural A, Mushtaq K, Zhang C, Alwakeel M, Sayles V, Duly K, Langford C, Hajj-Ali R. Efficacy and Safety Assessment of a Rituximab Biosimilar in Patients with Granulomatosis with Polyangiitis (GPA) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-assessment-of-a-rituximab-biosimilar-in-patients-with-granulomatosis-with-polyangiitis-gpa/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-assessment-of-a-rituximab-biosimilar-in-patients-with-granulomatosis-with-polyangiitis-gpa/