Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Inflammation of the synovium and in particular the bone marrow, as assessed by magnetic resonance imaging (MRI), have been identified as prognostic indicators of structural joint damage in patients (pts) with rheumatoid arthritis (RA).1 Tofacitinib is an oral Janus kinase (JAK) inhibitor for the treatment of RA. Inhibition of structural damage has been shown using conventional radiography in pts receiving tofacitinib for moderate to severe RA.2 Here we explore the effects of tofacitinib, with or without methotrexate (MTX), on tissue inflammation and progression of structural damage in early RA using highly-sensitive MRI endpoints.
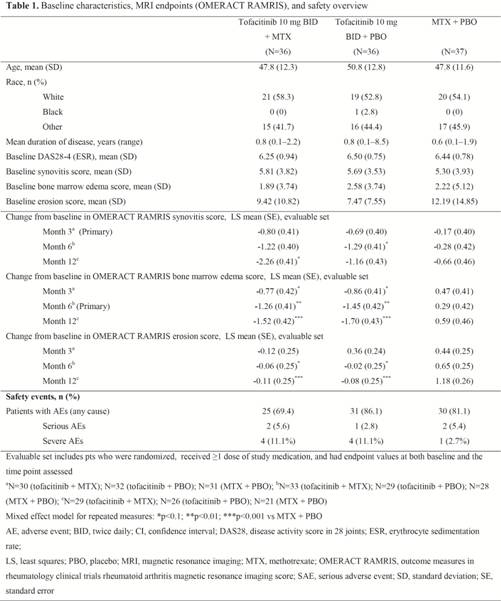
Methods: This was an exploratory, Phase 2, randomized, double-blind, parallel group, multicenter study (NCT01164579) in MTX-naïve adult pts with early active RA (duration ≤2 years) and evidence of clinical synovitis in an index wrist or metacarpophalangeal (MCP) joint. Pts were randomized 1:1:1 to receive tofacitinib 10 mg twice daily (BID) + MTX, tofacitinib 10 mg BID + placebo (PBO), or MTX + PBO, for 1 year. MTX was titrated, if tolerated, from 10 mg once weekly for the first month to 20 mg weekly by Month 2. Change in synovitis, bone marrow edema (BME), and erosions were assessed in the wrist and MCP joints using OMERACT RAMRIS. Co-primary endpoints: change from baseline (BL) in synovitis at Month (M) 3, and change from BL in BME at M6. Evaluable pts were assessed using a mixed effect model for repeated measures to evaluate endpoints (statistical significance at 10% [2-sided] level). Scoring was performed by one centralized reader blinded to time point and treatment.
Results: Of 109 pts randomized and treated (Table 1), most were female and Caucasian. Disease duration was consistent with early RA (Table 1). Mean age, disease activity, and RAMRIS were similar across treatment groups at BL (Table 1). More pts from the tofacitinib + MTX and tofacitinib + PBO groups completed the study (n=28, n=27, respectively) vs the MTX + PBO group (n=21). Synovitis improvements were observed in all groups; while improvements were numerically greater in both tofacitinib groups vs MTX + PBO, statistically significant differences were not consistently observed across assessment time points. Mean BME improvements were statistically greater in both tofacitinib groups vs MTX + PBO at M6 (primary), M3 and M12. Significantly less erosive damage was seen in both tofacitinib groups vs MTX + PBO at M6 and M12.
Conclusion: These results provide evidence of a reduction in tissue inflammation using assessments identified as positive prognostic factors for radiographic joint damage. The greater magnitude of improvement in BME and inhibition of erosive damage measured by MRI is consistent with the established effect of tofacitinib on inhibiting radiographic structural damage.
1. Palosaari K, et al. Rheumatology (Oxford) 2006; 45: 1542-8.
2. Lee EB, et al. N Engl J Med 2014;370: 2377-86.
Disclosure:
P. G. Conaghan,
Abbvie,
8,
Merck Pharmaceuticals,
8,
Novartis Pharmaceutical Corporation,
8,
Pfizer Inc,
8,
Roche Pharmaceuticals,
8,
UCB,
8,
Abbvie,
5,
Merck Pharmaceuticals,
5,
Novartis Pharmaceutical Corporation,
5,
Pfizer Inc,
5,
UCB,
5,
Roche Pharmaceuticals,
5;
M. Østergaard,
Abbott Laboratories,
2,
Centocor, Inc.,
2,
Merck Pharmaceuticals,
2,
Schering-Plough,
2,
Abbott Laboratories,
5,
Bristol-Myers Squibb,
2,
Boehringer Ingelheim,
5,
Eli Lilly and Company,
5,
Centocor, Inc.,
5,
GlaxoSmithKline,
5,
Janssen Pharmaceutica Product, L.P.,
5,
Merck Pharmaceuticals,
5,
Mundipharma,
5,
Navo,
5,
Pfizer Inc,
5,
Schering-Plough,
5,
Roche Pharmaceuticals,
5,
UCB,
5,
Wyeth Pharmaceuticals,
5;
C. Wu,
Bioclinica Inc,
3;
D. van der Heijde,
Pfizer Inc,
5;
F. Irazoque-Palazuelos,
Bristol-Myers Squibb,
9,
Pfizer Inc,
9,
UCB,
9,
Janssen Pharmaceutica Product, L.P.,
9,
Roche Pharmaceuticals,
9;
P. Hrycaj,
Pfizer Inc,
2,
Pfizer Inc,
5;
Z. Xie,
Pfizer Inc,
1,
Pfizer Inc,
3;
R. Zhang,
Pfizer Inc,
1,
Pfizer Inc,
3;
B. T. Wyman,
Pfizer Inc,
1;
J. D. Bradley,
Pfizer Inc,
1,
Pfizer Inc,
3;
K. Soma,
Pfizer Inc,
1,
Pfizer Inc,
3;
B. Wilkinson,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-tofacitinib-on-bone-marrow-edema-synovitis-and-erosive-damage-in-methotrexate-naive-patients-with-early-active-rheumatoid-arthritis-duration-%e2%89%a42-years-results-of-an-explora/