Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Cigarette smoking is a known risk factor for developing rheumatoid arthritis (RA).1 Several recent observational studies suggest that cigarette smoking may be associated with a poor response to treatment with methotrexate (MTX) and TNF inhibitors (TNFi).2,3 Tofacitinib is a novel, oral Janus kinase inhibitor for the treatment of RA. Analyses investigated the effects of smoking on the response to tofacitinib treatment in patients (pts) with RA enrolled in the Phase (P) 3 program.
Methods:
Data from five P3 studies of tofacitinib as monotherapy (ORAL Solo, NCT00814307) or in combination with MTX or other nonbiologic disease-modifying anti-rheumatic drugs (DMARDs) (ORAL Scan, NCT00847613 [1 year data]; ORAL Sync, NCT00856544; ORAL Standard, NCT00853385; ORAL Step, NCT00960440) in DMARD-inadequate responder pts were pooled and analyzed by smoking status at baseline (BL) for pts treated with tofacitinib 5 mg twice daily (BID), 10 mg BID and placebo (PBO). ORAL Standard included an adalimumab (40 mg Q2W; ADA) arm; data from the small subgroup of this population are presented for completeness.
Results:
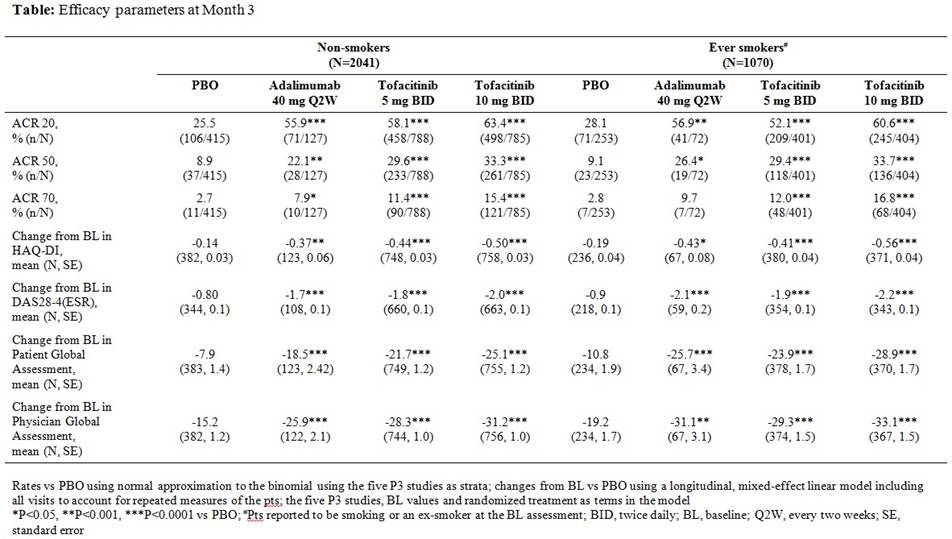
Of the 3315 pts included in the analysis, 1143 (34.5%) reported to be smoking or an ex-smoker (“ever” smokers) at BL. There were greater proportions of males (non-smokers 8.8%, ever smokers 30.7%) and white pts (non-smokers 52.9%, ever smokers 80.2%) among ever smokers; other BL demographics and disease characteristics were similar for non-smokers and ever smokers (age, BMI, RF status, anti-CCP status, DAS28-4(ESR) and HAQ-DI). A history of ever smoking at BL was associated with a higher probability of previous treatment failures with TNFi (non-smokers 19.0%, ever smokers 31.0%) for tofacitinib and PBO arms; a smaller proportion of ADA pts had previous exposure to TNFi regardless of smoking history (<10%). Significant and similar treatment differences for tofacitinib vs PBO for efficacy parameters at Month 3 were seen regardless of smoking status at BL (Table; significance declared as p≤0.05, 2-sided testing, no adjustment for multiple comparisons). Correlations calculated between smoking duration as of BL and Month 3 continuous efficacy measures (e.g. change from BL in Patient Global Assessment) indicated that smoking duration was generally uncorrelated with efficacy. No consistent differences across efficacy measures were observed between pts that never smoked, were ex-smokers or currently smoked at BL.
Conclusion:
This pooled analysis from five P3 trials indicates that pts receiving tofacitinib achieved a significant response vs PBO, regardless of smoking status at BL. Further research is required to examine the effect of smoking on response to tofacitinib over the long-term.
References:
1. Heliövaara M et al. J Rheumatol. 1993;20(11):1830-5
2. Saevarsdottir S et al. Arthritis Rheum. 2011;63(1):26-36
3. Canhão H et al. Rheumatology (Oxford). 2012;51(11):2020-6
Disclosure:
J. M. Kremer,
AZ, Genentech, Lilly, Pfizer, AZ,
2,
AZ, Genentech, Lilly, Pfizer, AZ,
5;
J. D. Greenberg,
Corrona,
1,
AstraZeneca, Corrona, Pfizer,
5;
C. Turesson,
Abbvie, Pfizer, Roche,
2,
Abbvie, BMS, Janssen, MSD, Pfizer, Roche, UCB,
5;
D. Gruben,
Pfizer Inc,
1,
Pfizer Inc,
3;
C. A. Mebus,
Pfizer Inc,
1,
Pfizer Inc,
3;
E. Bananis,
Pfizer Inc,
1,
Pfizer Inc,
3;
T. Robinson,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-smoking-status-on-response-to-treatment-with-tofacitinib-in-patients-with-rheumatoid-arthritis/