Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Upadacitinib (UPA) is an oral, selective Janus kinase (JAK)- inhibitor that has been shown to be effective and well-tolerated in patients with rheumatoid arthritis (RA) in the Phase 3 SELECT clinical trials and was approved in Canada as a treatment option for RA in December 2019. The Canadian Real-Life post-marketing Observational Study assessing the Effectiveness of UPadacitinib for treating rheumatoid arthritis (CLOSE-UP) study initiated in 2020 aims to investigate the effectiveness of UPA across 390 Canadians including csDMARD, bDMARD and tsDMARD experienced real-world RA patients.
Methods: CLOSE-UP is an ongoing, prospective, multicenter, observational post-marketing study in adults with moderate-to-severe RA who are treated with UPA 15 mg once daily, with the decision to initiate UPA made prior to study participation. Participants are followed for 24 months following UPA initiation with data collected at routine clinic visits. The primary endpoint is the proportion of participants achieving a Disease Activity Score – 28 Joint Count, C-reactive protein (DAS28-CRP) < 2.6 at 6 months. Secondary endpoints include, pain score using a visual analog scale, fatigue (FACIT-F), physical function as measured by the Health Assessment Questionnaire (HAQ) and other assessments of disease activity including Clinical Disease Activity Index (CDAI) score. Per protocol, eligible subjects are grouped by prior/most recent exposure to: no b/tsDMARDs (bio-naïve); ≤2 bDMARDs but no tsDMARD (bio-experienced), and ≤1 bDMARD followed by a tsDMARD (tsDMARD-experienced). This descriptive interim analysis reports data for participants who had completed their 6-month visit by March 18, 2022. Data are presented as observed and summarized descriptively, with no statistical analyses conducted.
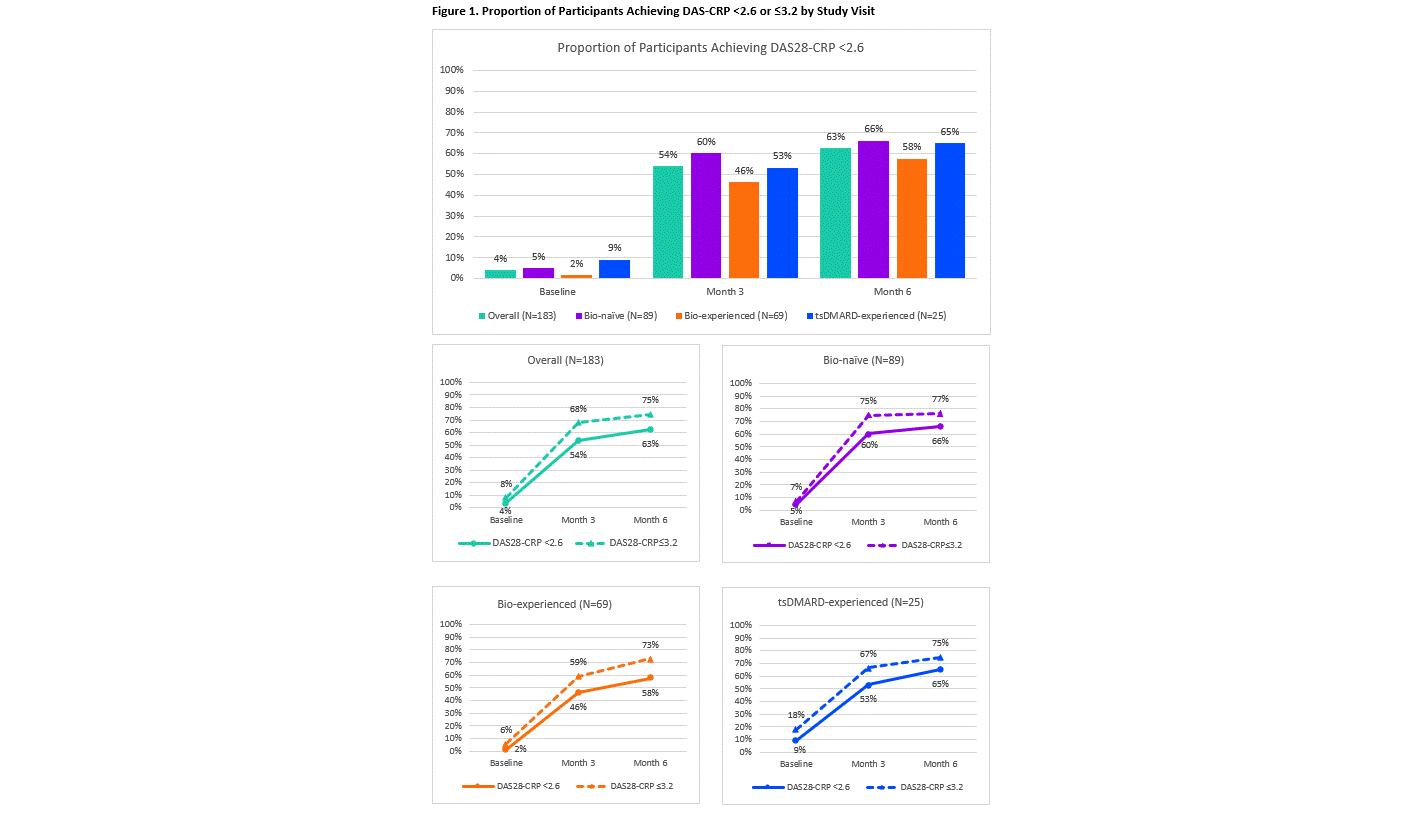
Results: Of the 183 participants included in this interim analysis, 89 (49%) were bio-naïve, 69 (38%) were bio-experienced and 25 (14%) were tsDMARD-experienced (Table 1). Overall, 63% of patients achieved a DAS28-CRP < 2.6 (primary endpoint; Figure 1) The proportion of participants achieving DAS28-CRP < 2.6 at the 6-month visit was similar between those receiving UPA monotherapy (62.2%, n=69/111) and those receiving UPA in combination with a csDMARD (63.9%, n=36/45). Physical function and patient reported outcomes including pain and fatigue improved over the first 6 months following UPA initiation. The safety profile of UPA was consistent with that seen in Phase 3 trials with no new safety signals (Table 2).
Conclusion: Consistent with clinical trial data, interim analysis of this real-world Canadian study showed that disease activity was reduced, and patient reported outcomes improved with an overall favorable benefit:risk profile for Canadian patients receiving UPA in the real-world setting.
2. Has not been previously exposed to tsDMARD and has been previously exposed to ≤2 bDMARDs.
3. Has been previously treated with one tsDMARD and ≤1 bDMARD prior to treatment with that tsDMARD.
For readability, parameters with no missing data do not have patient numbers specified. Cardiovascular risk factors include Smoking, BMI>=30, hypertension, diabetes, atherosclerosis, ischemic heart disease.
APCA, anti-citrullinated protein antibody; b, biologic; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; cs, conventional synthetic; DAS28, Disease Activity Score – 28 Joint Count; DMARD, disease modifying antirheumatic drug; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate; RF, rheumatoid factor; TJC, tender joint count; ts, targeted synthetic; SJC, swollen joint count; VAS, visual analog scale.
To cite this abstract in AMA style:
Haaland D, Chan J, Lisnevskaia L, Chow A, Girard T, Fournier P, Bessette L. Effectiveness of Upadacitinib in Patients with Rheumatoid Arthritis in Canadian Real-World Practice: Interim Results from the CLOSE-UP Post-Marketing Observational Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-patients-with-rheumatoid-arthritis-in-canadian-real-world-practice-interim-results-from-the-close-up-post-marketing-observational-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-patients-with-rheumatoid-arthritis-in-canadian-real-world-practice-interim-results-from-the-close-up-post-marketing-observational-study/