Session Information
Date: Sunday, November 8, 2020
Title: Epidemiology & Public Health II: Risk Factors & Outcomes (1467–1471)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Arthritis often leads to presenteeism (decreased at-work productivity) and permanent work disability, the worst occupational outcome of a disease, leading to reduced quality of life and cost to society. Yet, health services addressing employment needs of people with arthritis are lacking. We evaluated the effectiveness of the Making-it-WorkTM (MIW), an online program developed to help people with inflammatory arthritis (IA) deal with employment issues.
Methods: A multi-center RCT evaluated the effectiveness of MIW at improving presenteeism and work cessation (WC) over two years. Participants were recruited from rheumatologist practices, consumer organizations and arthritis programs, in three provinces. Eligibility criteria: diagnosis of IA, employed, age 18-59, and concerned about ability to work. Participants were randomized 1:1 to MIW or usual care plus printed material on workplace tips. MIW consists of five online self-learning modules and group meetings, and individual vocational counselling and ergonomic assessments. Questionnaires were administered every 6 months. Outcomes were presenteeism [Rheumatoid Arthritis Work Instability Scale (RA-WIS)], time to WC of ≥6 months duration, and time to WC ≥2 months (secondary outcome). Baseline characteristics (age, gender, ethnicity, occupation, education, disease duration and self-employment) were collected. Intention-to-treat longitudinal analysis of RA-WIS using linear mixed effect regression models with 2-year comparison as primary endpoint and survival analysis for time to WC using Kaplan-Meier and Cox Proportional Hazard models were performed. Sensitivity analyses were conducted with missing values imputed using last observation carried forward and worse possible outcomes; with square root transformation of RA-WIS outcome; and adjusting for baseline covariates. SAS version 9.4 was used.
Results: A total of 564 participants were recruited, with 85% completing 2-year follow-up. Baseline characteristics were similar between groups. Difference in means of RA-WIS scores was significantly lower in the intervention group from 6 months onwards, with the greatest difference observed at 2 years (-1.78, 95%CI: -2.7, -0.9, p< .0001), yielding a standardized effect size of 0.32. Sensitivity analysis revealed satisfactory robustness of results. Work cessation occurred less often in intervention than control groups, but only reached statistical significance for WC duration ≥2 months ( WC ≥6 months: 31 vs 44 events, aHR 0.70, 95%CI: 0.44, 1.11, p-value: 0.13; WC ≥2 months: 39 vs 61 events, aHR: 0.65, 95%CI: 0.43, 0.98, p-value: 0.04).
Conclusion: Results of the RCT reveal the program was effective at improving presenteeism and preventing short-term WC. Effectiveness at preventing long-term work disability will be assessed at 5 years. This program fills one of the most important unmet needs for people with inflammatory arthritis.
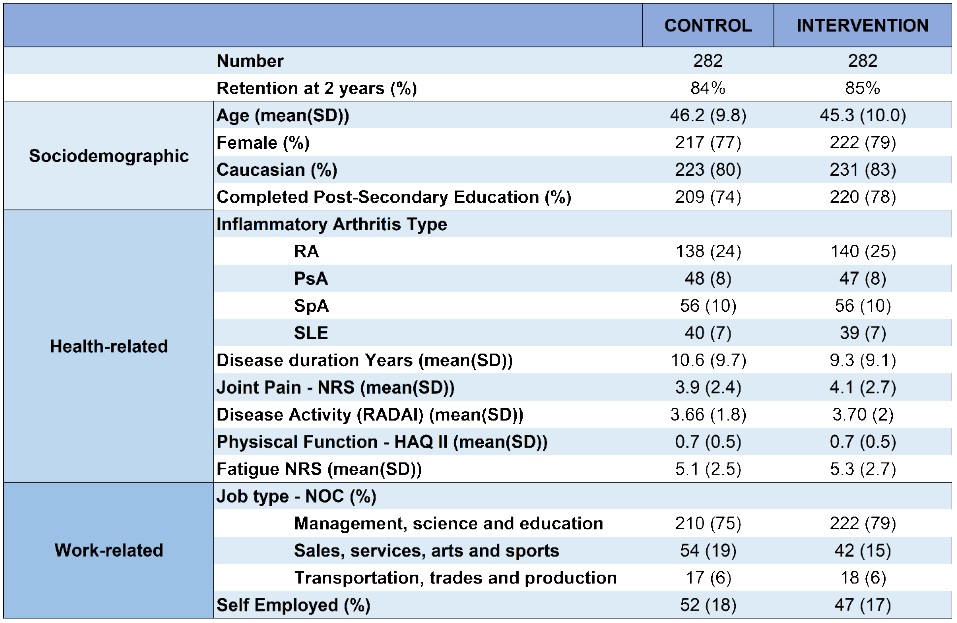 Table 1. Sample characteristics
Table 1. Sample characteristics
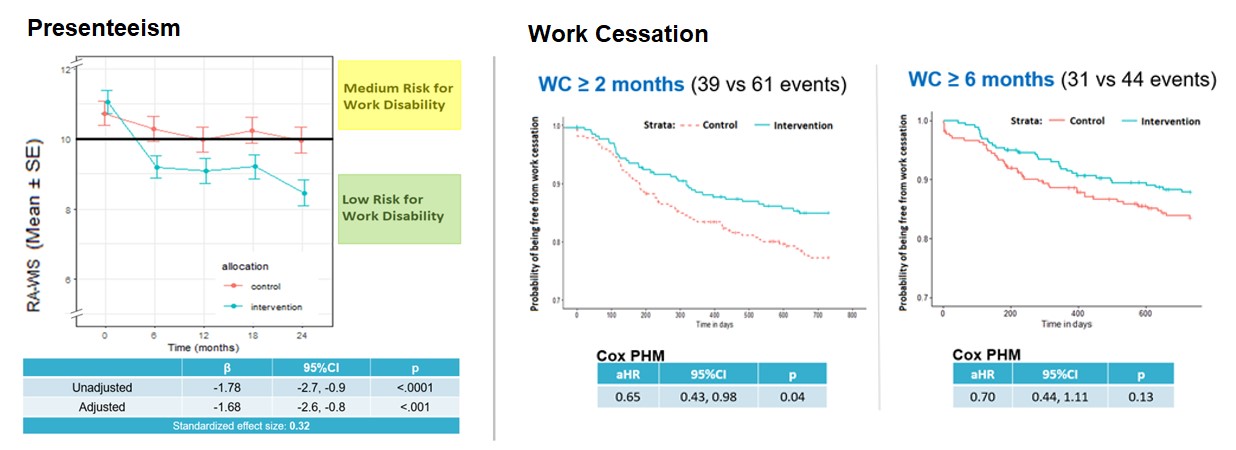 Figure 1. Effectiveness of the Making-it-Work(TM) program on Presenteeism and Work Cessation over 2 years
Figure 1. Effectiveness of the Making-it-Work(TM) program on Presenteeism and Work Cessation over 2 years
To cite this abstract in AMA style:
Luquini A, Zheng Y, Xie H, Backman C, Rogers P, Kwok A, Knight A, Gignac M, Mosher D, Li L, Esdaile J, Thorne C, Lacaille D. Effectiveness of the Making It Work™ Program at Improving Presenteeism and Work Cessation in Workers with Inflammatory Arthritis – Results of a Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effectiveness-of-the-making-it-work-program-at-improving-presenteeism-and-work-cessation-in-workers-with-inflammatory-arthritis-results-of-a-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-the-making-it-work-program-at-improving-presenteeism-and-work-cessation-in-workers-with-inflammatory-arthritis-results-of-a-randomized-controlled-trial/
