Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite extensive data supporting the effectiveness of mRNA vaccines against SARS-CoV-2 in immunocompetent individuals, their efficacy in immunosuppressed patients remains uncertain. Consequently, this study aims to assess the immune response to the SARS-CoV-2 mRNA vaccine in patients with rheumatologic diseases who are on immunosuppressive therapy.
Methods: This retrospective case series compiled data for 339 patients with rheumatologic conditions on immunosuppressants and 87 healthy controls. The most prevalent rheumatologic conditions were rheumatoid arthritis (n = 69, 20.4%), osteoarthritis (n = 53, 15.6%), systemic lupus erythematosus (n = 40, 11.8%), and psoriasis/psoriatic arthritis (n = 16, 4.7%). Data collected included patient demographics, diagnosis history, immunosuppressive medications, and anti-SARS-CoV-2 spike protein antibody titers. Antibodies against SARS-CoV-2 were measured using a commercial quantitative anti-SARS-CoV-2 spike protein antibody immunoassay (Roche).
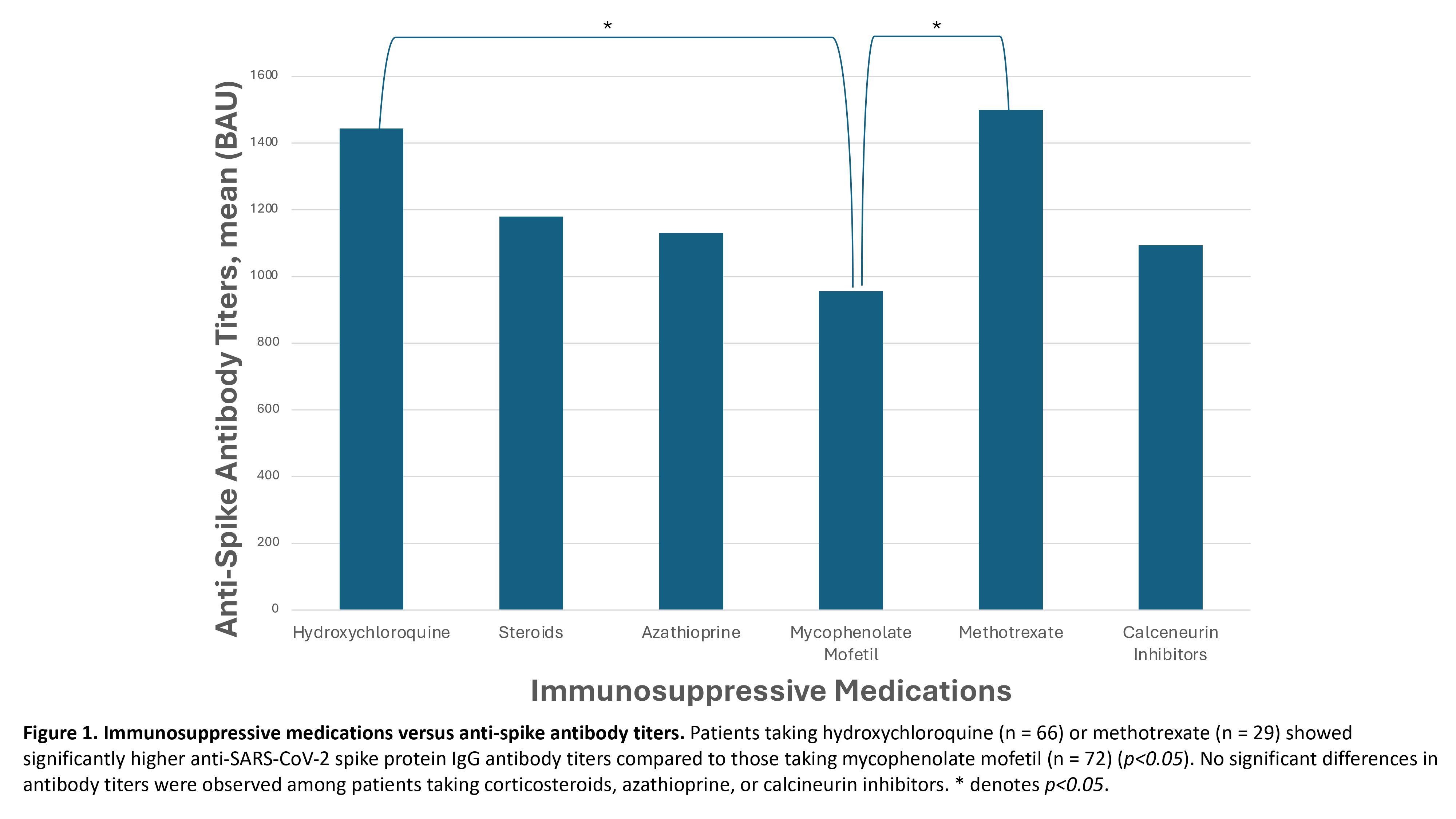
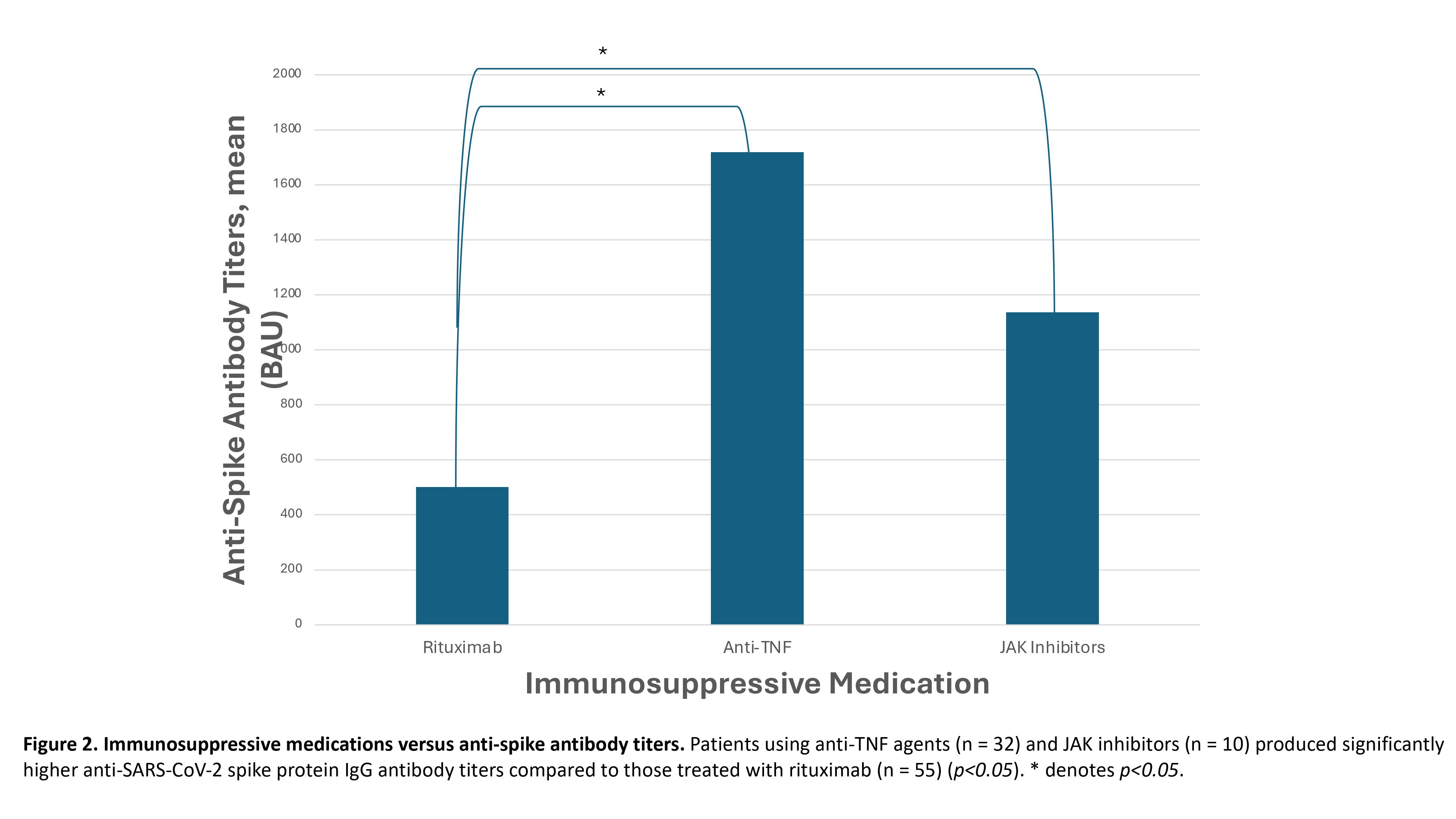
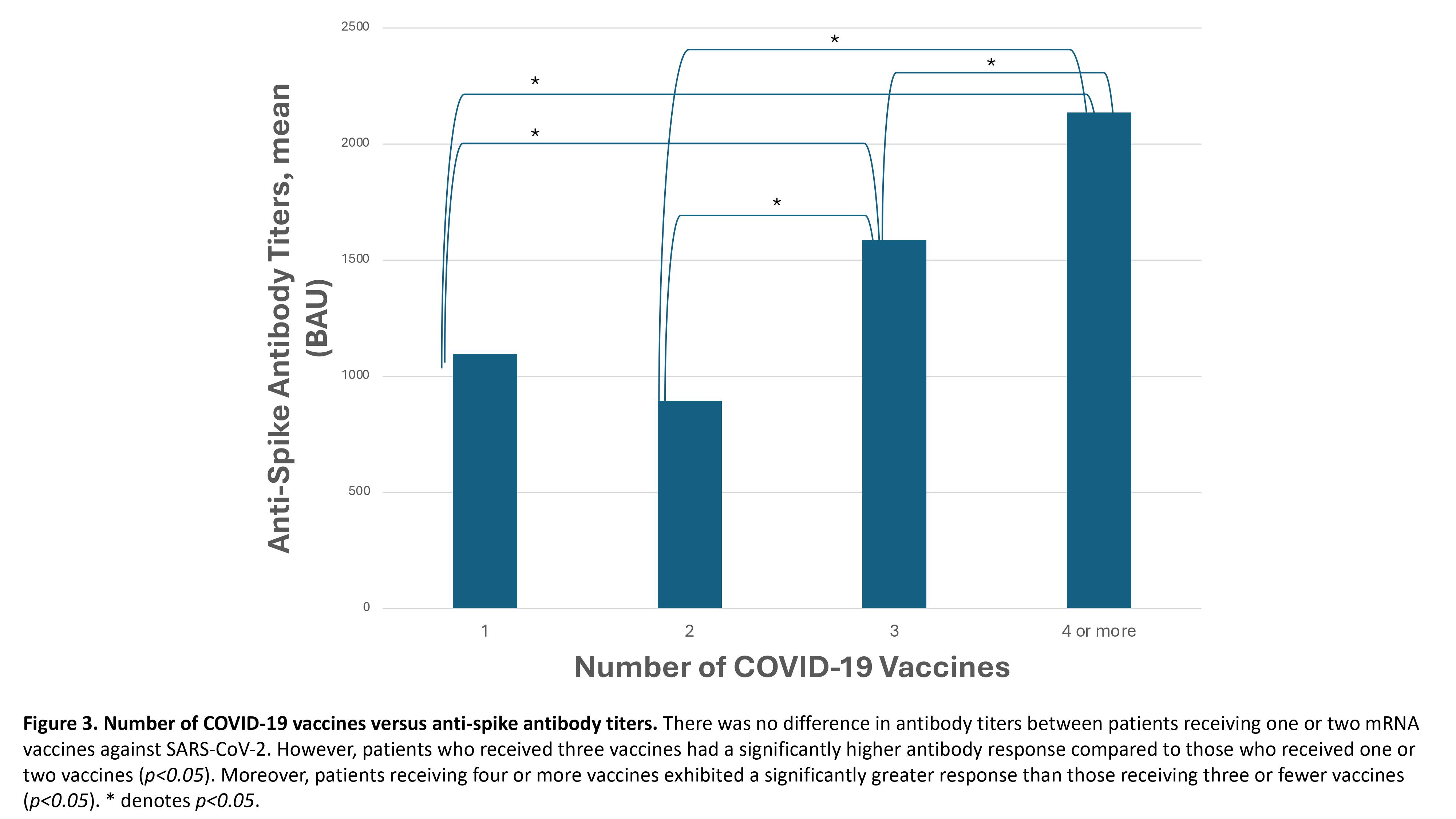
Results: As expected, patients with rheumatologic conditions on immunosuppressants demonstrated significantly lower levels of anti-SARS-CoV-2 spike protein IgG antibodies compared to healthy controls (p< 0.05). Patients taking hydroxychloroquine (n = 66) or methotrexate (n = 29) showed significantly higher anti-SARS-CoV-2 spike protein IgG antibody titers compared to those taking mycophenolate mofetil (n = 72) (Figure 1, p< 0.05). No significant differences in antibody titers were observed among patients taking corticosteroids, azathioprine, or calcineurin inhibitors. Additionally, patients using anti-TNF agents (n = 32) and JAK inhibitors (n = 10) produced significantly higher antibody titers compared to those treated with rituximab (n = 55) (Figure 2, p< 0.05). Patients using a single immunosuppressive agent (n = 172) exhibited significantly higher antibody titers than those using two or more immunosuppressive agents (n = 167) (p< 0.05). Finally, there was no difference in antibody titers between patients receiving one or two mRNA vaccines against SARS-CoV-2 (Figure 3). However, patients who received three vaccines had a significantly higher antibody response compared to those who received one or two vaccines (Figure 3, p< 0.05). Moreover, patients receiving four or more vaccines exhibited a significantly greater response than those receiving three or fewer vaccines (Figure 3, p< 0.05).
Conclusion: This study highlights that patients with rheumatologic conditions on immunosuppressants exhibit a reduced ability to generate an immune response to mRNA vaccines against SARS-CoV-2 compared to healthy controls. Notably, those taking mycophenolate mofetil or rituximab, as well as any patient on two or more immunosuppressive agents, show an especially decreased capacity to mount an immune response. Consequently, it is advisable to minimize the number of immunosuppressants these patients are on whenever possible. Moreover, significant increases in anti-SARS-CoV-2 spike protein antibody titers are observed after three or more vaccine doses, indicating that two or fewer doses are insufficient for this immunosuppressed population.
To cite this abstract in AMA style:
Levian B, Botz C, Chwa J, Grove S, Ravi V, Ireifej B, arkfeld d, Hanna D, Hu J, Ngo B. Effectiveness of mRNA COVID-19 Vaccines in Patients with Rheumatologic Conditions on Immunosuppressive Therapies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-mrna-covid-19-vaccines-in-patients-with-rheumatologic-conditions-on-immunosuppressive-therapies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-mrna-covid-19-vaccines-in-patients-with-rheumatologic-conditions-on-immunosuppressive-therapies/