Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Medication non-adherence in rheumatoid arthritis (RA) is associated with disease flares, increased disability and increased costs. Electronic Monitoring Feedback (EMF) to improve adherence has shown promising effects in various chronic diseases, however, it has never been studied in RA nor with biological DMARDs. This study aims to assess the effectiveness of EMF on medication adherence in RA patients starting with or switching to a new bDMARD.
Methods: In this RCT, RA patients starting a (new) bDMARD were 1:1 assigned to the intervention or control group and followed for 1 year. The intervention group received a special needle container which was equipped with a Medication Event Monitoring System (MEMS) cap registering patient’s adherence to subcutaneous medication injections. During the intervention, adherence score was read out every 3 months with MEMS, on which motivational interviewing feedback based on patient’s medication adherence was given. Furthermore, reasons for non-adherence were explored and counselled. The control group received usual care. At the start and after 12 months, Compliance Questionnaire in Rheumatology (CQR), Beliefs about Medicines Questionnaire (BMQ) and Health Assessment Questionnaire (HAQ) were obtained. The feedback group also received BMQ and HAQ at months 3, 6 and 9. Effectiveness of EMF on adherence was assessed with the validated discriminant CQR-Z score for taking compliance ≤80% and medication possession ratio (MPR, total day’s supply in period divided by total period, truncated to 1.0). Patients taking >80% of their medication were considered adherent. Effectiveness of EMF on DAS28-ESR and side effects was also assessed.
Results: 207 consecutive patients were included,105 in feedback group and 102 control (mean age 52 years, 74% female, 88% Caucasian ethnicity, 5.3 years since diagnosis of RA, 51 used a bDMARD before). At baselins there were no differences with respect to the DAS28 and HAQ (intervention 3.95 vs control 3.74 and 1.15 vs 0.98 respectively) in both groups. Most patients started with etanercept (95) or adalimumab (91).
The percentage of CQR adherent patients increased for both groups from 68.5 and 63.1 (baseline) to 75.6 and 81.7 (12 months) for intervention and controls respectively (no difference between groups at baseline and 12 months, p=0.534 and p=0.446.
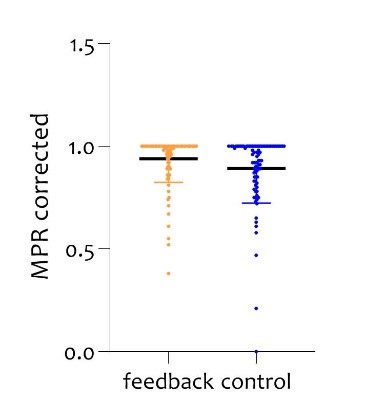
MPR was 0.95 in the feedback group and 0.92 in the control group (p=0.048, Figure 1), corrected for returned defect syringes and medical indicated postponing
Correct dosing measured by MEMS decreased slightly from 92.6% (after 3 months) to 90.5% (after 12 months).
Methotrexate, prednisolone and other csDMARD use throughout the study was similar for both groups and BMQ and HAQ scores remained nearly equal.
Side effects such as infections did not differ between groups (47% and 57% of the patients reported any side effect, p=0.142).
Conclusion: Taking compliance and correct dosing were relatively high in RA patients starting with or switching to a biological DMARD, as measured with MPR, CQR and MEMS. Since high rates were found in the control group too, probably because of a ceiling and a placebo effect, EMF did not increase adherence. Presumably the effect of EMF should be targeted to non-adherent patients.
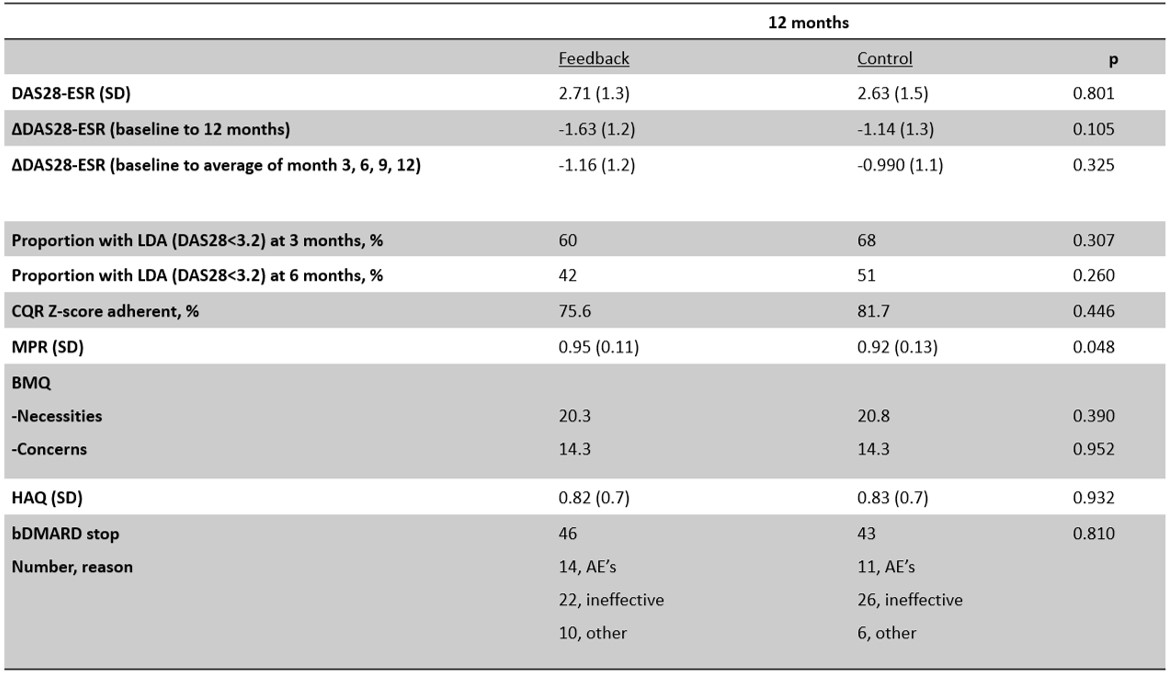 Table 1. results for both groups. Means and number of patients are presented, unless otherwise stated. CQR Zk- score combined with DAS score was available for 64 patients.
Table 1. results for both groups. Means and number of patients are presented, unless otherwise stated. CQR Zk- score combined with DAS score was available for 64 patients.
To cite this abstract in AMA style:
Hebing R, Bos W, Nurmohamed M, van den Bemt B. Effectiveness of Electronic Drug Monitoring Feedback in Order to Increase Adherence in RA Patients Starting with a Biological DMARD [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effectiveness-of-electronic-drug-monitoring-feedback-in-order-to-increase-adherence-in-ra-patients-starting-with-a-biological-dmard/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-electronic-drug-monitoring-feedback-in-order-to-increase-adherence-in-ra-patients-starting-with-a-biological-dmard/