Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: PsA
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Tumor Necrosis Factor inhibitors (TNFi) are effective in PsA and axial SpA, but are associated with increased infections risk, patient burden and high costs. The DRESS-PS study1 was the first study to show that a Treat-To-Target (T2T) tapering strategy in PsA and axSpA patients is non-inferior compared to T2T without tapering regarding Low Disease Activity (LDA) status at 12 months, with a considerable reduction of TNFi use. If these effects can be maintained up to 2 years is unknown.
The aim of this study was to describe effectiveness of disease activity guided dose optimization of TNFi up to 24 months follow-up in patients with PsA and axSpA who participated in the DRESS-PS study.
Methods: This study is a 12-month observational extension of the DRESS-PS study. In the DRESS-PS study, patients were allocated to protocolized T2T tapering (intervention) or to T2T without tapering (control). During the extended follow-up, treatment decisions were according to usual care and T2T tapering was allowed for all patients. Patients visited the outpatient clinic bi-annually.
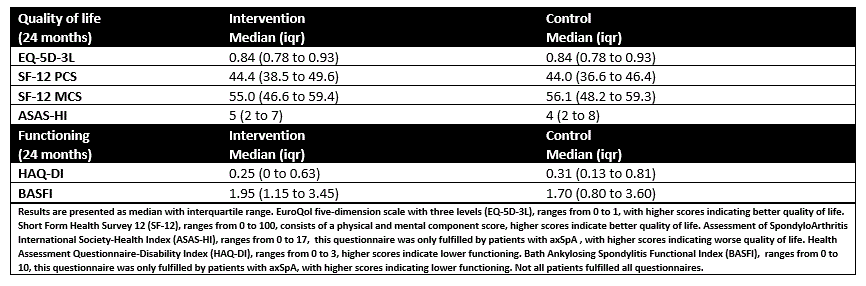
Primary outcomes were disease activity (presented as proportion in LDA and mean disease activity), and TNFi use (presented as percentage of the current daily dose to the defined daily dose (DDD)). Secondarily, functioning measured with HAQ-DI and BASFI and quality of life measured with EQ5D, SF12 and ASAS-HI at 24 months are reported.
We also compared effects of protocolized tapering to routine care tapering, by comparing the intervention group at 12 months to the control group at 24 months.
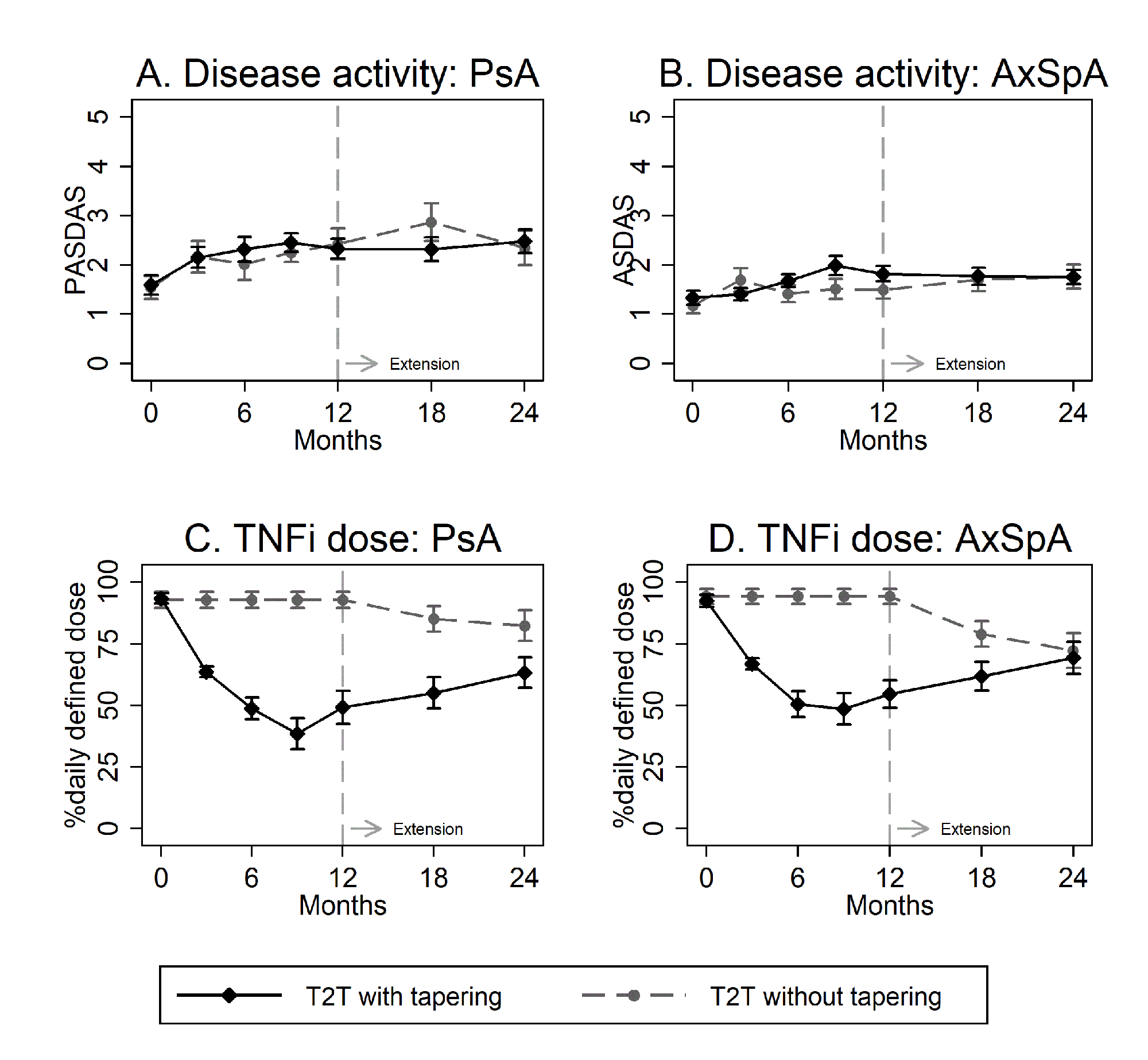
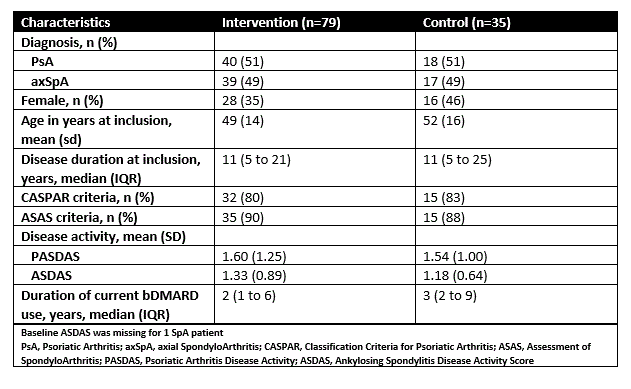
Results: 114 patients (of 122) were included in the extension study (table 1). The proportion in LDA state at 24 months was 67% (45 of 67) in patients originally allocated in the intervention group and 72% (23 of 32) in patients originally allocated in the control group (difference 4.7% (95%CI: -15% to 24%)). Mean disease activity remained stable during the extension period (Figure 1A and 1B).
101/114 (89%) patients attempted tapering during follow up (100% intervention vs 63% control). TNFi %DDD at 24 months was 66% (95%CI: 57% to 75%) in the original intervention group and 77%% (95%CI: 68% to 87%) in the original control group (Figure 1C and D). Functioning and quality of life at 24 months was similar between patients originally randomized to intervention and control (table 2).
The comparison between protocolized tapering and routine care tapering showed no difference in patients in LDA state 12 months after start of tapering: 70% vs 72%% (difference 2.3% (95%CI: -16% to 21%)). However, there was a significant difference in TNFi use: %DDD 52% in the intervention group at 12 months and 77% in the control group at 24 months (difference 26% (95%CI: -13% to 38%)).
Conclusion: T2T tapering of TNFi remains effective and safe regarding disease control up to two years. However, the %DDD seems to increase somewhat in the intervention group, and is higher compared to studies in RA, and tapering in usual care seems less frequent. Whether this is by chance, or caused by a disease-specific need for higher dosing or more subjective disease activity measures, remains a topic for further research.
References
1. Michielsens CA et al. Ann Rheum Dis. 2022 Oct;81(10):1392-1399.
To cite this abstract in AMA style:
Peeters A, Michielsens C, Mahler E, Verhoef L, den Broeder A, den Broeder N, van Herwaarden N. Effectiveness of Dose Reduction and Withdrawal Strategies of TNF Inhibitors in Psoriatic Arthritis and Axial Spondyloarthritis: Long Term Extension of the DRESS-PS Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-dose-reduction-and-withdrawal-strategies-of-tnf-inhibitors-in-psoriatic-arthritis-and-axial-spondyloarthritis-long-term-extension-of-the-dress-ps-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-dose-reduction-and-withdrawal-strategies-of-tnf-inhibitors-in-psoriatic-arthritis-and-axial-spondyloarthritis-long-term-extension-of-the-dress-ps-study/