Session Information
Date: Monday, November 8, 2021
Title: Abstracts: RA – Treatments II: New Findings in Established Therapies (1442–1445)
Session Type: Abstract Session
Session Time: 2:00PM-2:15PM
Background/Purpose: With the arrival of new Janus kinase inhibitors (JAKi), with different JAK inhibition profiles, there is the possibility of using a second JAKi in the event of failure to a first JAKi in patients with rheumatoid arthritis (RA). There are no data on the effectiveness of cycling JAKi compared to switching to biologic disease-modifying antirheumatic drug (bDMARD) in patients who have failed a first JAKi. The objective of this study is to compare the effectiveness of cycling JAKi vs switching to bDMARD in a real-world RA population.
Methods: Nested cohort study within an international collaboration of RA registries (data contributed from 14 national registries of the JAK-pot collaboration). We pooled prospectively collected data from RA patients who failed a first JAKi and were subsequently treated with either a second JAKi (JAKi cycling) or with a bDMARD (switching) in routine care. We compared the effectiveness of both strategies in terms of drug retention and in terms of disease activity (DAS28) evolution over 1 year after second treatment initiation. Differences in drug survival rates were assessed by Log Rank Test. DAS28 trajectories were predicted based on an age- and gender-adjusted linear mixed model with a quadratic trend over time.
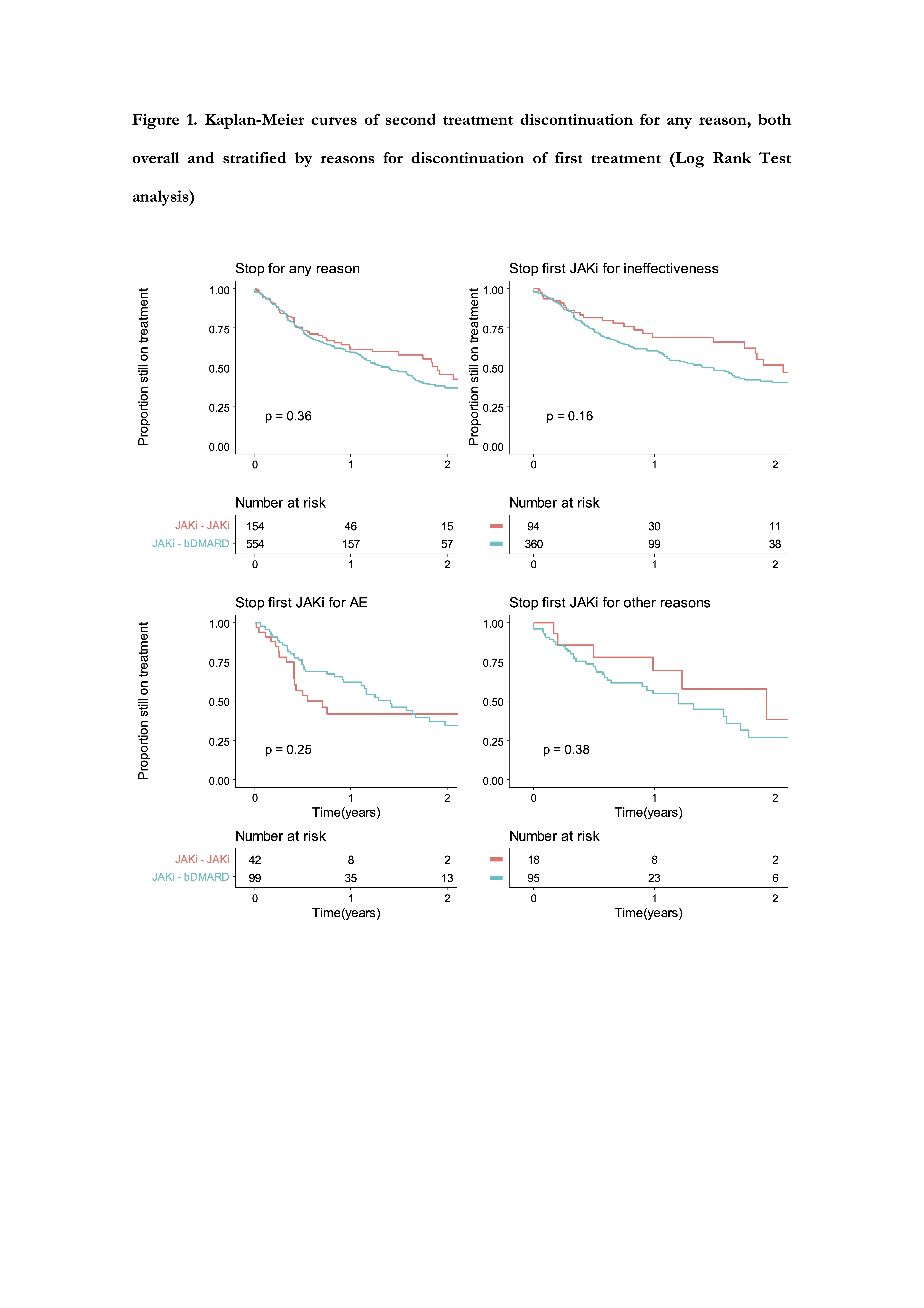
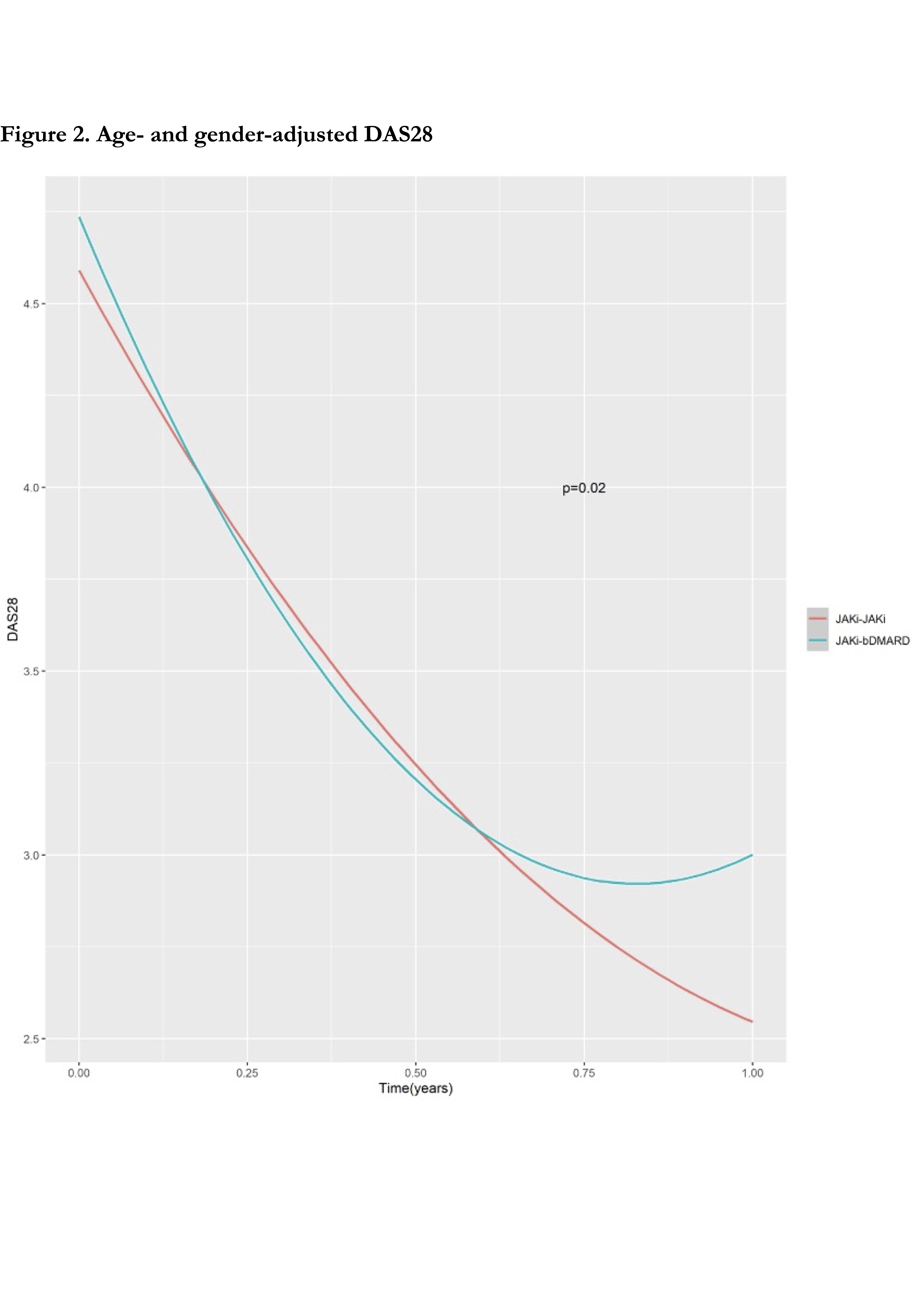
Results: 708 patients who failed JAKi were included. 154 cycled to a second JAKi and 554 switched to a bDMARD (Table 1). Patients cycling JAKi were older, had longer disease duration, had received more bDMARDs and had longer exposure to first JAKi treatment than switchers to a bDMARD. Monotherapy was more prevalent and discontinuation of the first JAKi was more common for safety reasons than for lack of effectiveness. Cycling and switching strategies showed similar drug survival rates after two years of follow-up (Figure 1). Nevertheless, a non-significant trend emerged where discontinuation was more likely among patients cycling JAKi when reason for stopping the first JAKi was an adverse event, whereas discontinuation was less likely among patients cycling JAKi when reason for stopping the first JAKi was ineffectiveness. DAS28 over time evolved in a similar way between patients cycling JAKi and switching to a bDMARD, with improvements after one year of follow-up (Figure 2).
Conclusion: After failing a first JAKi, cycling JAKi versus switching to a bDMARD appears to have similar effectiveness despite a more difficult to treat patient profile for the patients cycling to JAKi.
To cite this abstract in AMA style:
Pombo-Suarez M, Sanchez-Piedra C, Gomez-Reino J, Lauper K, Inanc N, Strangfeld A, Huschek D, Pavelka K, Kristianslund E, Kvien T, Rotar Z, Nordström D, Choquette D, Elkayam O, Leeb B, José Santos M, Hyrich K, Kearsley-Fleet L, Codreanu C, Monguin D, Courvoisier D, Finckh A. Effectiveness of Cycling JAKi Compared to Switching to bDMARD in Patients Who Failed a First JAKi in an International Collaboration of Registries of Rheumatoid Arthritis Patients (the JAK-pot Study) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-cycling-jaki-compared-to-switching-to-bdmard-in-patients-who-failed-a-first-jaki-in-an-international-collaboration-of-registries-of-rheumatoid-arthritis-patients-the-jak-pot-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-cycling-jaki-compared-to-switching-to-bdmard-in-patients-who-failed-a-first-jaki-in-an-international-collaboration-of-registries-of-rheumatoid-arthritis-patients-the-jak-pot-study/