Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Long-term treatment with abatacept, a selective T-cell co-stimulation modulator, is effective and well tolerated in patients with JIA.1–4 The objective of this analysis was to provide real-world data on the longitudinal effectiveness of intravenous (IV) and subcutaneous (SC) abatacept in patients with JIA, classified by category, enrolled in the Pediatric Rheumatology Collaborative Study Group (PRCSG)/Paediatric Rheumatology INternational Trials Organisation (PRINTO) registry.
Methods: Following a standardized protocol and data collection process, clinical sites across 23 countries enrolled patients with JIA receiving/initiating IV or SC abatacept with a planned 10-year duration of follow-up. Treatment response was assessed at 3, 6, 9, 12, 24, 36, 48, and 60 months, and was evaluated in this analysis by clinical 10-joint Juvenile Arthritis Disease Activity Score (cJADAS10). The cJADAS10 used validated cut-offs for low disease activity (LDA; ≤3.8), inactive disease (ID; ≤ 1.0)5, and remission (ID for ≥ 6 months). An as-observed analysis is presented.
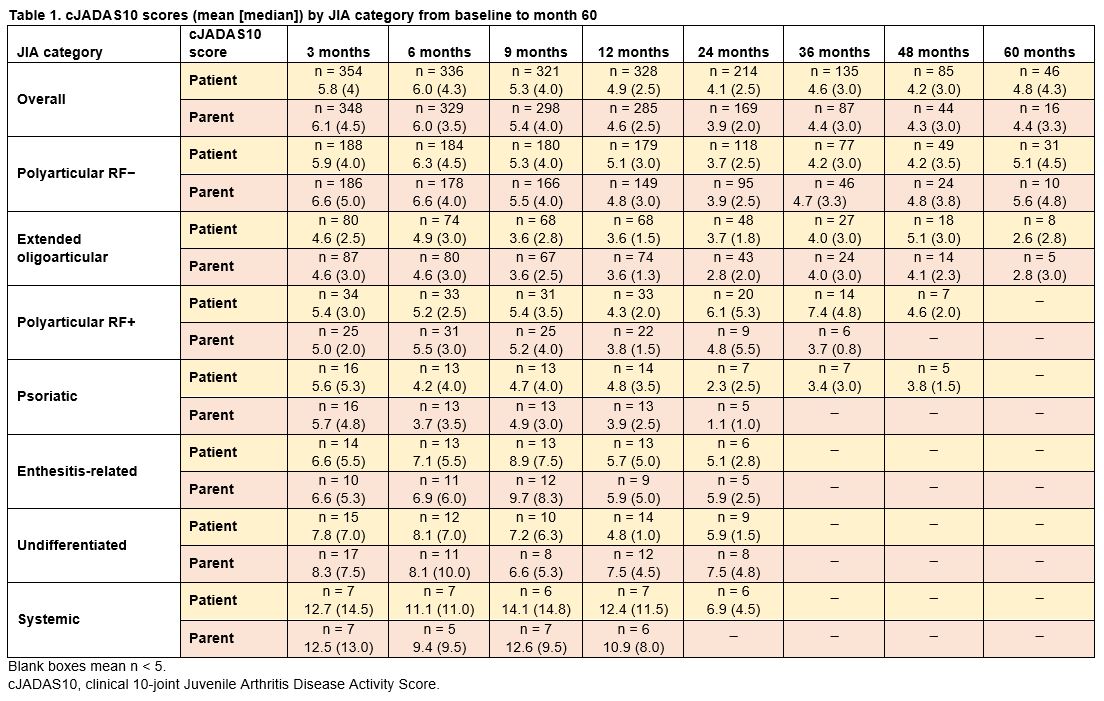
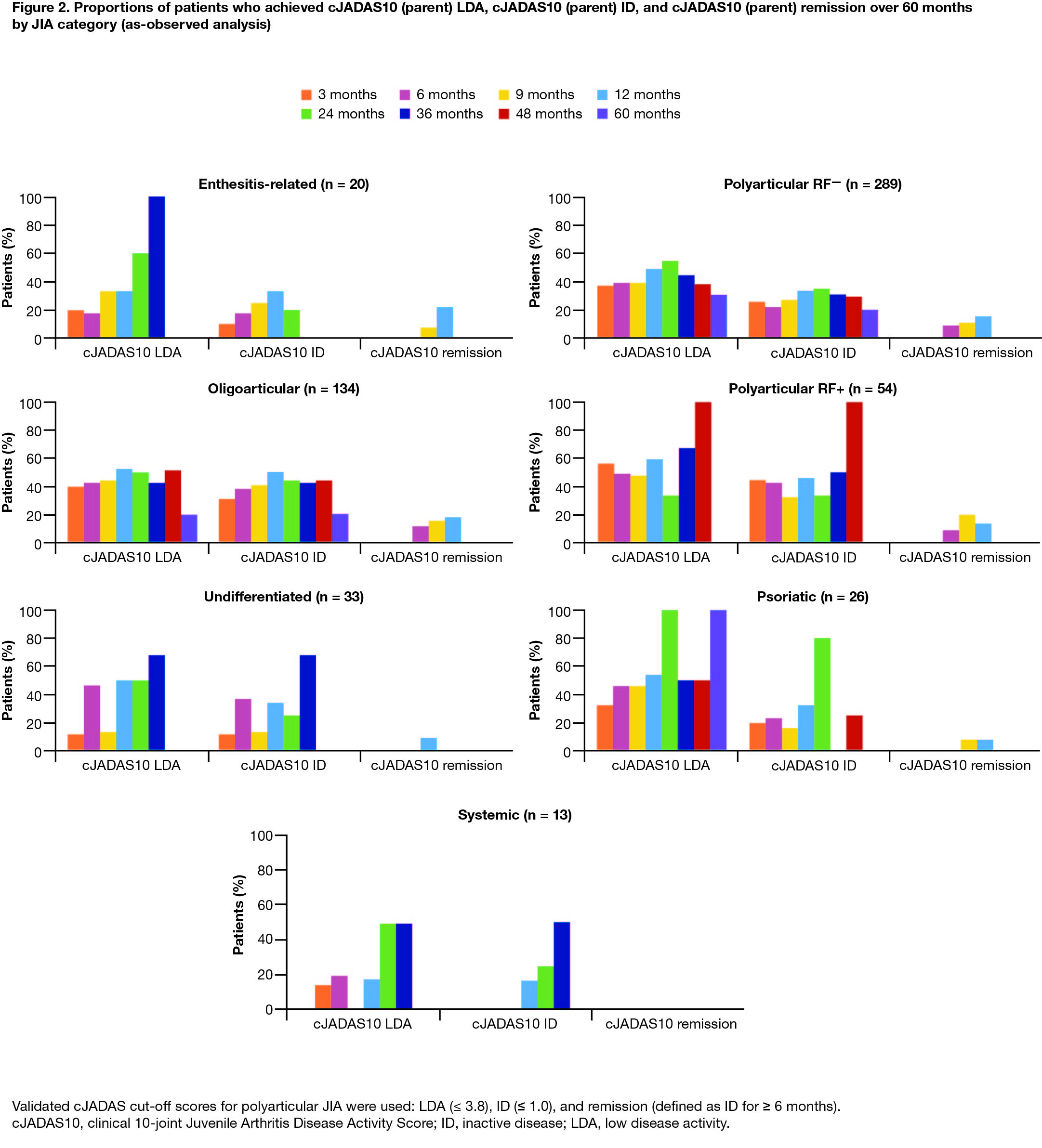
Results: As of March 31, 2020, 569 patients were enrolled in the PRCSG/PRINTO registry; 454 (79.8%) were female. Mean age at enrollment was 13.1 years, mean time since JIA diagnosis was 66.1 months, and mean abatacept treatment duration was 13.1 months. Half of the patients (n = 289; 50.8%) had polyarticular RF− JIA and a quarter (n = 134; 23.6%) had extended oligoarticular JIA; other enrolled patients had polyarticular RF+ (n = 54; 9.5%), psoriatic (n = 26; 4.6%), enthesitis-related (n = 20; 3.5%), undifferentiated (n = 33; 5.8%), and systemic (n = 13; 2.3%) JIA. The treatment response (patient and parent cJADAS10 score) by JIA category from baseline to month 60 is shown in Table 1. The change in mean cJADAS10 score over time by JIA category (Figure 1) demonstrates a general trend towards reduced score over time. The proportions of patients achieving cJADAS10 LDA and ID by JIA category were generally improved or sustained over time (Figure 2). The low patient numbers in some JIA categories at later time points were mainly due to patients being lost to follow-up or not having reached those time points due to ongoing enrollment. At 60 months, 30% and 20% of patients with polyarticular RF− JIA (most prevalent category) had achieved and maintained cJADAS10 LDA and ID, respectively. Overall, 14.7% of patients achieved cJADAS10 remission by month 12; similar trends (7–22%) were seen in most JIA categories.
Conclusion: A proportion of patients in each JIA category achieved cJADAS10 LDA and ID. As sample size decreased over time in some JIA categories, these data should be interpreted with caution. Clinically important and sustained responses with abatacept, generally up to 36 and 48 months, were seen in all JIA categories.
References: 1. Brunner HI, et al. Arthritis Rheumatol 2018;70:1144–1154. 2. Ruperto N, et al. Lancet 2008;372:383–391. 3. Lovell DJ, et al. Arthritis Rheumatol 2015;67:2759–2770. 4. Ruperto N, et al. Arthritis Rheumatol 2010;62:1792–1802. 5. Consolaro A, et al. Arthritis Care Res (Hoboken) 2014;66:1703–1709.
Medical writing: Fiona Boswell, PhD (Caudex), funded by Bristol Myers Squibb
To cite this abstract in AMA style:
Lovell D, Tzaribachev N, Ting T, Alexeeva E, Giani T, Griffin T, Bohnsack J, Zeft A, Vehe R, Tarvin S, Horneff G, Trachana M, Dominique A, Dong L, Kou T, Wong R, Martini A, Brunner H, Ruperto N. Effectiveness of Abatacept in Patients with JIA, Classified by Category: Results from the PRCSG/PRINTO JIA Real-World Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-abatacept-in-patients-with-jia-classified-by-category-results-from-the-prcsg-printo-jia-real-world-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-abatacept-in-patients-with-jia-classified-by-category-results-from-the-prcsg-printo-jia-real-world-registry/