Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Oral urate-lowering therapy (ULT) is one of the primary treatments for gout. Unfortunately, a proportion of patients with advanced gout are resistant to oral ULT or have severe tophaceous gout. For patients with uncontrolled gout, pegloticase, a recombinant pegylated uricase, is approved by the US Food and Drug Administration (FDA) and has proven efficacy. However, pegloticase use can be limited by immunogenicity as the development of anti-drug antibodies contributes to both loss of efficacy and a higher risk of infusion reactions. Small clinical trials (Khanna, 2021; Boston, 2021) have suggested that concomitant immunomodulatory (IMM) drugs (e.g. mycophenolate moefitil or methotrexate) reduce pegloticase immunogenicity and improve treatment persistence, but real-world evidence is still lacking. Therefore, we evaluated pegloticase persistence and adverse events associated with concomitant IMM drug use in patients with uncontrolled gout.
Methods: We conducted a retrospective cohort and selected patients with procedure code(s) for pegloticase (J2507) using ACR’s Rheumatology Informatics System for Effectiveness (RISE) registry from 01/2016 through 06/2020. The first date of pegloticase was defined as the index date. Based on use of the concomitant IMM drug, we identified 2 exposure groups: 1) IMM users (defined as ≥ 1 IMM prescription within ±60days of index); 2) non-IMM users. We calculated the proportion of patients who ever achieved the serum urate (sUA) ≤ 6mg/dL and patients who had lab abnormalities (WBC < 3.4; platelets < 135,000; hematocrit [HCT] < 30; ALT or AST ≥1.5X ULN) within 180 days after the index date. Time to pegloticase discontinuation was analyzed using cox regression model controlling for potential confounders including age, sex, race, body mass index (BMI), national area deprivation index (ADI), concurrent medications (Rx), and number of RxRisk categories (a measure of comorbidity based on medications for specific diseases).
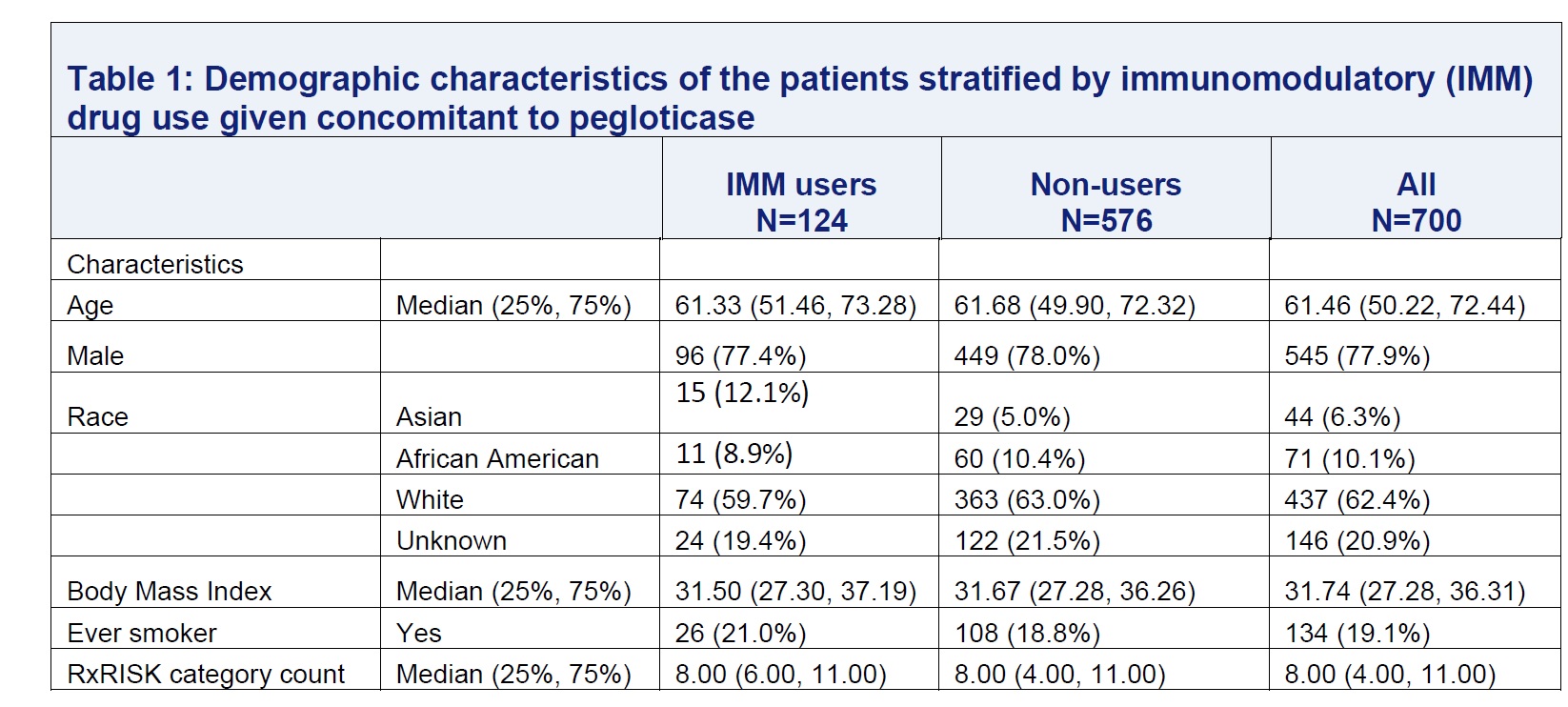
Results: We identified 700 pegloticase users with median follow-up of 14 months. Among these, 124 were IMM users and 576 non-users (Table 1). For IMM users, the most used IMM medications were methotrexate (79%), followed by azathioprine (12.1%). During follow-up, 90% of patients ever met sUA treatment target. The median number of pegloticase infusions were 7 for IMM drug users and 5 for non-users . Compared to non-users, IMM users were less likely to discontinue pegloticase (Figure 1). After adjustment, the hazard ratio of discontinuation of pegloticase associated with concomitant IMM therapy was 0.57 (95% CI: 0.43-0.78). Lab abnormalities were uncommon (< 5%) among pegloticase users, and were not higher in patients also on IMM therapy.
Conclusion: Consistent with small trials, results from this large observational registry suggest that concomitant immunomodulatory drug use improves pegloticase persistence. Rare lab abnormalities suggest no disproportionate toxicity resulting from this strategy.
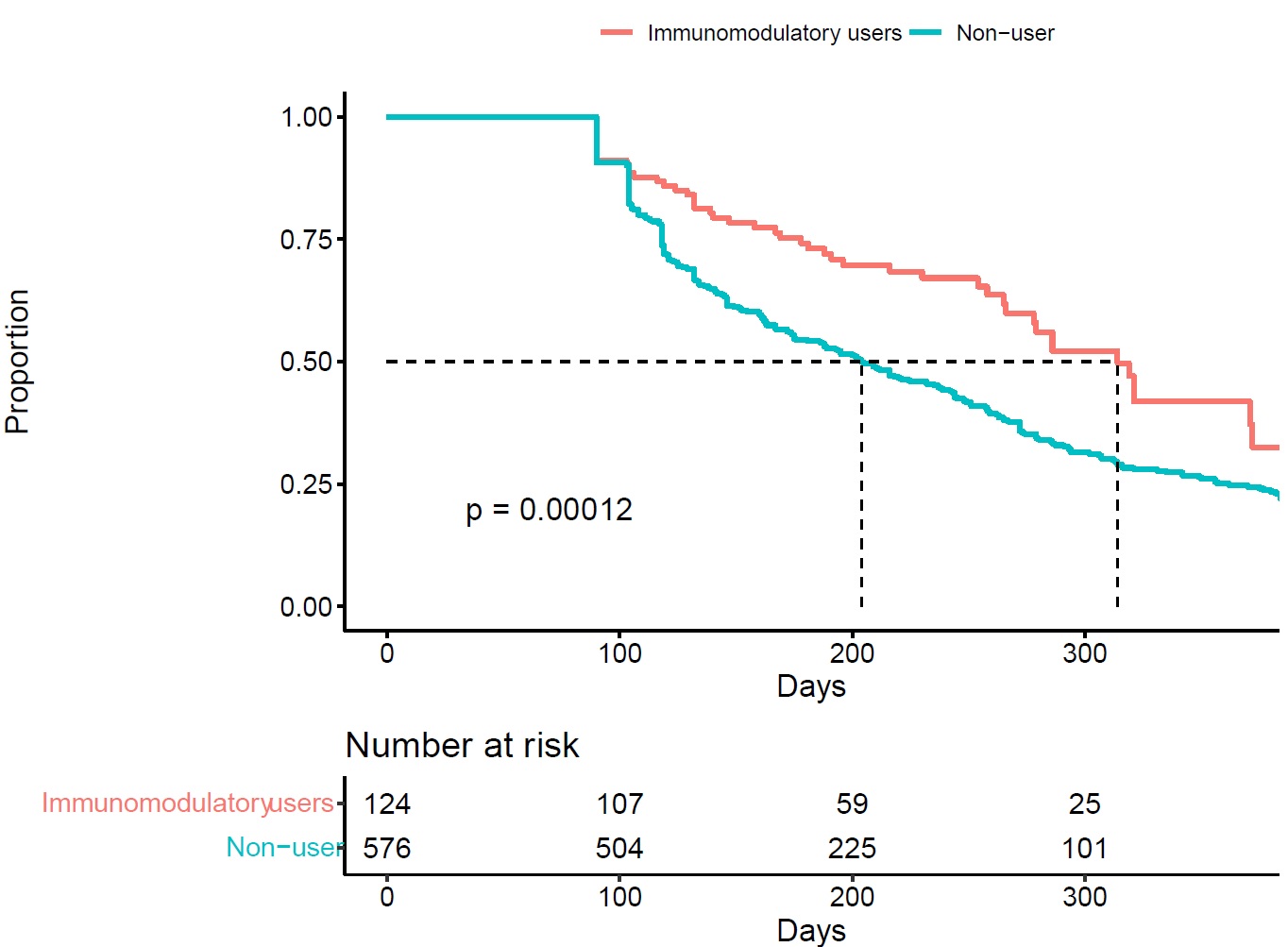 Figure 1: Kaplan-Meier plot for time to discontinuation stratified by immunomodulatory drug use given concomitant to pegloticase
Figure 1: Kaplan-Meier plot for time to discontinuation stratified by immunomodulatory drug use given concomitant to pegloticase
To cite this abstract in AMA style:
Yun H, LaMoreaux B, Chen L, Ledbetter S, Francis-Sedlak M, Saag K, Mikuls T, Curtis J. Effectiveness and Safety of Pegloticase with Concomitant Immunomodulatory Therapy [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-pegloticase-with-concomitant-immunomodulatory-therapy/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-pegloticase-with-concomitant-immunomodulatory-therapy/