Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Iguratimod (IGU), an oral synthetic disease-modifying antirheumatic drug (DMARD), has shown significant efficacy and safety in rheumatoid arthritis (RA) when combined with methotrexate (MTX) or as monotherapy in Japanese studies [Ishiguro et al, 2013]. RA is a chronic autoimmune disease that, without early intervention, leads to irreversible joint damage and disability. Prompt initiation of DMARDs is crucial to achieving remission and preventing complications. Despite IGU’s therapeutic benefits, no randomized controlled trial (RCT) has evaluated its role in Indian RA patients. This study aims to assess the efficacy and safety of IGU with MTX in newly diagnosed, DMARD-naïve Indian patients with active RA.
Methods: In this randomized, double-blind, placebo-controlled, single-centre trial, eligible Indian patients (≥18 years, either gender) with DMARD-naïve, early active RA (ACR-EULAR 2010 criteria, DAS28-ESR ≥3.2, disease duration < 1 year) were randomized 1:1 to receive either IGU (25 mg twice daily) plus MTX or placebo plus MTX. MTX was initiated at 20mg/week for 12weeks and increased to 25mg/week for the next 12weeks. Randomization was performed using computer-generated block randomization. The primary endpoint was the proportion of patients achieving remission or low disease activity (LDA) per DAS28-ESR at 12 and 24 weeks. Secondary endpoints included ACR20/50/70 response rates at 24 weeks and incidence of adverse events. Statistical analysis was performed using GraphPad Prism v10, with p< 0.05 considered significant.
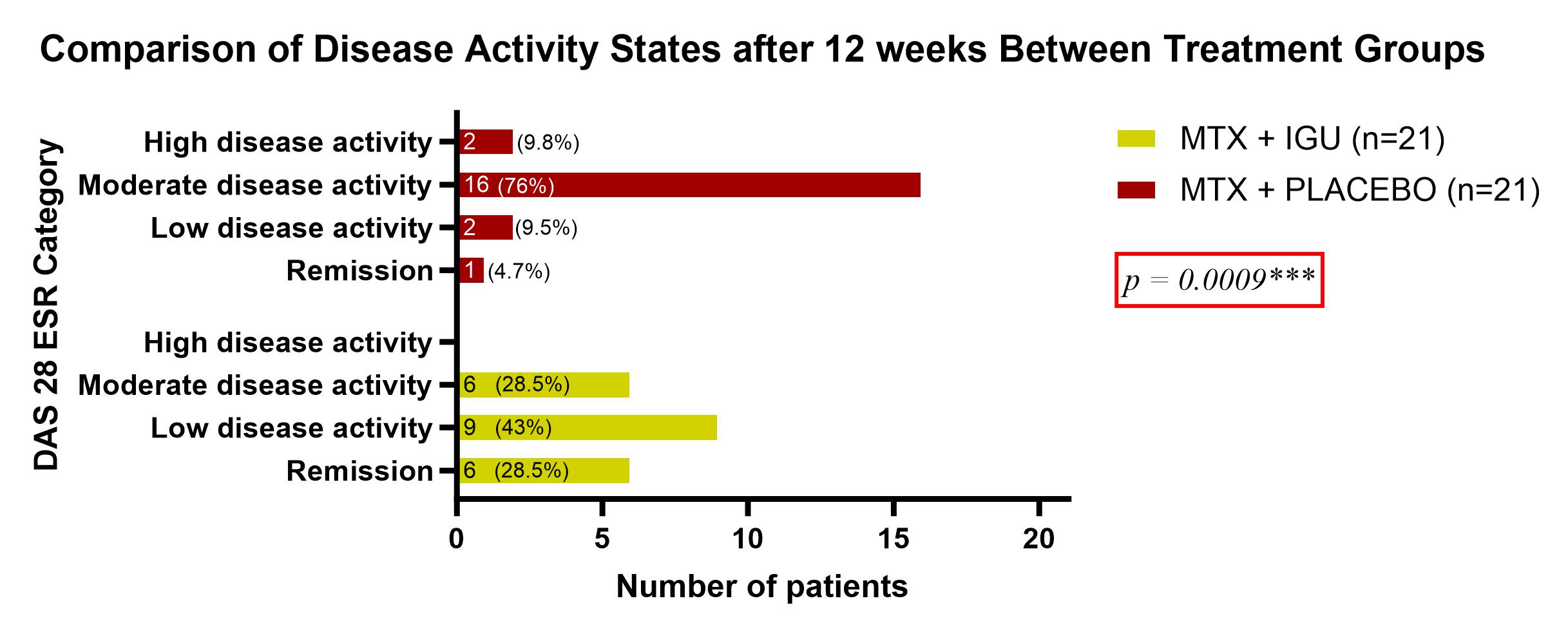
Results: A total of 42 patients were randomized and treated. Baseline characteristics were comparable: mean age was 49±12 years (test) and 48±10 years (placebo); females constituted 90% and 86%, respectively. High disease activity was observed in 90% (test) and 95% (placebo). At 12weeks, remission or LDA was achieved in 71% of the test group (28% remission, 43% LDA) versus 14% (5% remission, 9% LDA) in the placebo group (p=0.0009). By 24weeks, 100% in the test group (81% remission, 19% LDA) and 57% in the placebo group (14% remission, 43% LDA) achieved remission or LDA (p< 0.0001). ACR20/50/70 responses were significantly higher in the test group (100%, 88%, 59%) than in the placebo group (71%, 57%, 9%) (p< 0.0001). Adverse events were comparable (test: 5, placebo: 4, p >0.99), including leukopenia, transaminitis, GI symptoms, and mouth ulcers.
Conclusion: Iguratimod plus methotrexate demonstrated superior efficacy and safety in newly diagnosed DMARD-naïve Indian RA patients, with incremental benefits over placebo for remission or LDA (57% at 12weeks, 43% at 24weeks) and ACR responses (ACR20:29%, ACR50:31%, ACR70:50%). These findings align with Japanese studies [Ishiguro et al, 2013].
To cite this abstract in AMA style:
JALANATHAN M, Ali Mirza M, Parimi V, S S, Ramineni T. Effectiveness and Safety of Iguratimod with Background Methotrexate Therapy in Indian Patients with Rheumatoid Arthritis: A Randomized Double Blinded Placebo Controlled Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-iguratimod-with-background-methotrexate-therapy-in-indian-patients-with-rheumatoid-arthritis-a-randomized-double-blinded-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-iguratimod-with-background-methotrexate-therapy-in-indian-patients-with-rheumatoid-arthritis-a-randomized-double-blinded-placebo-controlled-study/

.jpg)