Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Recent
studies suggest that patients’ (pts’) body weight impacts response to anti-TNF
treatment in pts with spondyloarthropathies1 including axial
spondyloarthritis (axSpA).2 Here we investigate whether efficacy of
certolizumab pegol (CZP), a PEGylated Fc-free
anti-TNF, is affected by weight in axSpA pts.
Methods:
RAPID-axSpA (NCT01087762) was double-blind and placebo
(PBO)-controlled to Week (Wk) 24. Pts fulfilled ASAS
criteria and had active axSpA. At baseline (BL), pts were randomized 1:1:1 to
PBO, or CZP 200 mg Q2W/CZP 400 mg Q4W. The primary endpoint was the Wk12 ASAS20
response.3 In this post-hoc analysis, data from the overall
population were summarized by subgroups according to median BL weight (80 kg);
further analyses considered BL weight ≥100 kg. Efficacy by BL BMI (<25
kg/m2, normal weight; ≥25–<30 kg/m2, overweight;
≥30 kg/m2, obese) was also explored. Missing ASAS data were
imputed as non-responders; LOCF was used for continuous measures.
Results:
Of 325 pts randomized, 218 received CZP from Wk0, and
107 PBO. BL characteristics, including disease activity measures, were similar
between populations, though more males weighed ≥80 kg (76.5% male vs
46.2% male in <80 kg group) and CRP was higher in heavier pts (median CRP [mg/L]:
pts <80 kg, 11.5; pts ≥80 kg, 14.5; pts ≥100 kg, 15.0). At BL, pt numbers between BMI categories were similar (normal
weight: 114; overweight: 111; obese: 94).
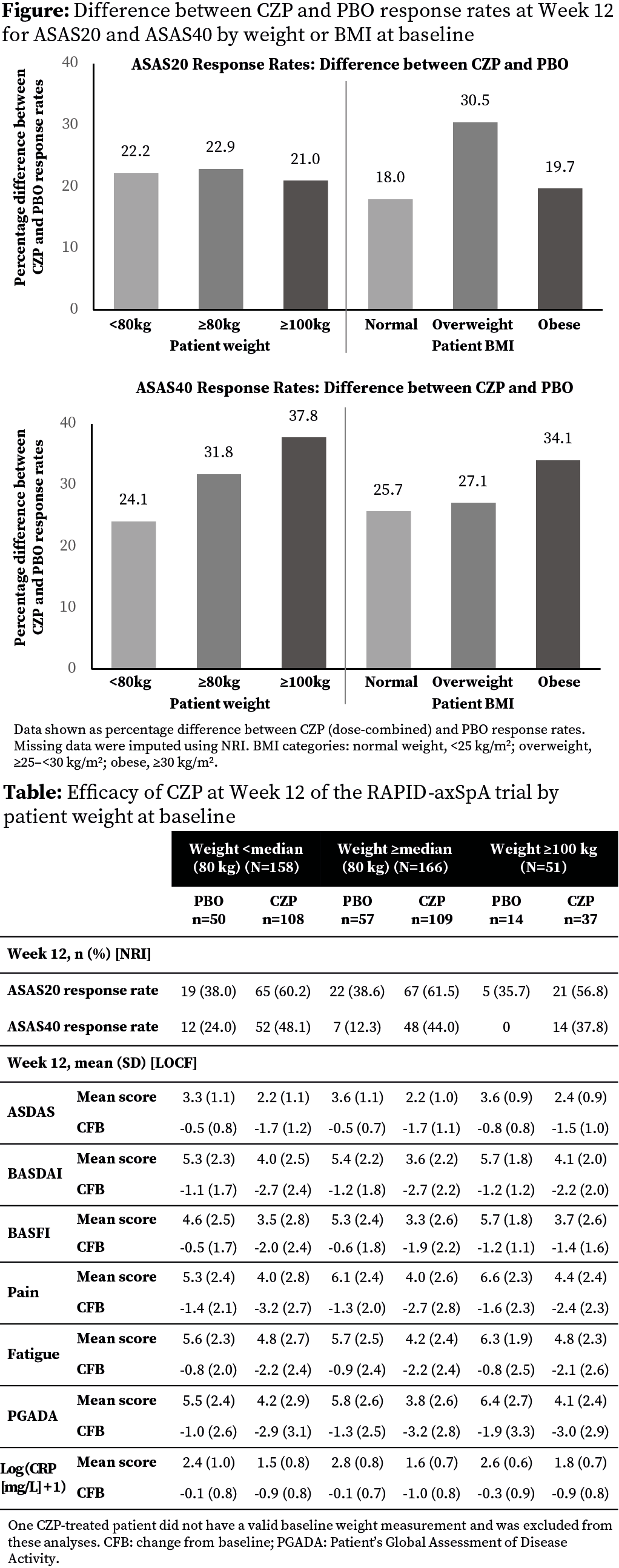
At Wk12, ASAS20 and ASAS40 response rates and
treatment differences were similar regardless of BL weight (Table, Figure).
Similarly, there was no decreasing trend in Wk12 response rates or treatment
differences for ASAS20 and ASAS40 across increasing BMI categories (Figure; CZP
dose combined: ASAS20, normal weight: 56.3% CZP [n=80] vs 38.2% PBO [n=34];
overweight: 72.6% CZP [n=73] vs 42.1% PBO [n=38]; obese: 55.0% CZP [n=60] vs
35.3% PBO [n=34]; ASAS40, normal weight: 46.3% CZP vs 20.6% PBO; overweight:
53.4% CZP vs 26.3% PBO; obese: 40.0% CZP vs 5.9% PBO).
Comparable improvements with CZP treatment were
observed across weight categories in clinical and pt-reported
outcomes, as well as laboratory measures of inflammation (Table); though pts
weighing ≥100 kg had lower BASFI improvements, possibly as a result of
the independent effect of weight on function.
Similar efficacy was maintained to Wk24 regardless of pt weight, with similar outcomes observed with both CZP
dose regimens (data not shown).
Conclusion:
In RAPID-axSpA, improvements to Wk12 of CZP treatment
in composite clinical outcomes, pt-reported and
laboratory outcome measures were similar regardless of pt
weight. These results were maintained over 24 wks of
CZP treatment with both CZP dose regimens.
References:
1. Ferraccioli G. Arthritis
Rheum 2010;62(S10):297
2. Gremese E. Rheumatology
2014;53:875–81
3. Landewé R. Ann Rheum Dis 2014;73:39–47
To cite this abstract in AMA style:
Deodhar AA, Sieper J, Davies O, Nurminen T, Mease PJ. Effect of Weight on Efficacy of Certolizumab Pegol in Patients with Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/effect-of-weight-on-efficacy-of-certolizumab-pegol-in-patients-with-axial-spondyloarthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-weight-on-efficacy-of-certolizumab-pegol-in-patients-with-axial-spondyloarthritis/