Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA), which has also been evaluated in other inflammatory rheumatic diseases (IRD) including psoriatic arthritis (PsA) and ankylosing spondylitis (AS). Pain contributes substantial morbidity in patients (pts) with IRD and directly impacts treatment adherence, assessment of disease improvement, and health-related quality of life. We evaluated the effectiveness of tofacitinib in reducing pain in randomized controlled clinical trials in pts with RA, PsA, and AS.
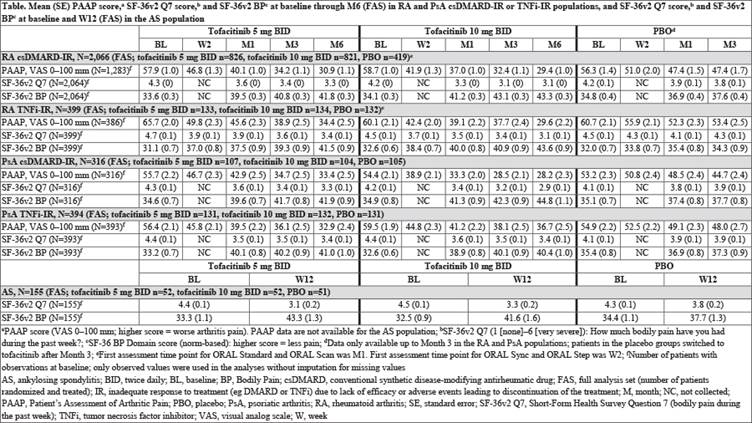
Methods: Five pt populations treated with tofacitinib 5 mg twice daily (BID), 10 mg BID, or placebo (PBO) were evaluated: [1] conventional synthetic disease-modifying antirheumatic drug (csDMARD)-inadequate response (IR) RA pts pooled from ORAL Scan (NCT00847613), ORAL Sync (NCT00856544), and ORAL Standard (NCT00853385), [2] tumor necrosis factor inhibitor (TNFi)-IR RA pts from ORAL Step (NCT00960440), [3] csDMARD-IR PsA pts from OPAL Broaden (NCT01877668), [4] TNFi-IR PsA pts from OPAL Beyond (NCT01882439), and [5] AS pts from a Phase 2 study (NCT01786668). Pain outcomes evaluated from baseline to Month (M)6 (Week [W]12 in the AS population) included Pt’s Assessment of Arthritis Pain (PAAP) (RA and PsA populations only), Short-Form Health Survey (SF)-36v2 Q7 (bodily pain in the past week), SF-36v2 Bodily Pain Domain (BP), EuroQol Five Dimensions Questionnaire Pain/Discomfort Domain (EQ-5D PD; all populations), and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) Q2 (level of AS neck, back, or hip pain) and Q3 (other pain) score (PsA and AS populations only; PsA pts had presence of spondylitis at screening and baseline BASDAI total score >0 in the full analysis set [FAS]). Data were analyzed descriptively.
Results: The csDMARD-IR RA, TNFi-IR RA, csDMARD-IR PsA, TNFi-IR PsA, and AS populations comprised a total of 2066, 399, 316, 394, and 155 pts in the FAS, respectively. In each RA or PsA csDMARD-IR and TNFi-IR population treated with tofacitinib, mean PAAP at baseline (5 mg BID, range 55.7–65.7 mm; 10 mg BID, 54.4–60.1 mm) decreased as early as W2 (1st post-baseline assessment; 45.8–49.8 mm; 38.9–44.8 mm) and continued to decrease through M6 (30.9–34.4 mm; 28.2–36.7 mm); decreases were numerically greater vs PBO and the magnitude of change in RA and PsA populations was similar (Table). Improvements in SF-36v2 Q7 (Table), SF-36v2 BP (Table), and EQ-5D PD were observed in all 4 RA and PsA csDMARD-IR and TNFi-IR populations, and in BASDAI Q2 and Q3 in the csDMARD-IR PsA and TNFi-IR PsA populations. In the AS population, improvements from baseline in mean SF-36v2 Q7 (Table), SF-36v2 BP (Table), EQ-5D PD, and BASDAI Q2 and Q3 were reported at W12 and were numerically greater vs PBO.
Conclusion: Treatment with tofacitinib is associated with a rapid improvement and sustained reduction of pain in pts with RA and PsA who are csDMARD-IR or TNFi-IR, and in pts with AS.
To cite this abstract in AMA style:
Ogdie A, de Vlam K, McInnes IB, Mease PJ, Baer P, Lukic T, Kwok K, Wang C, Hsu MA, Maniccia A. Effect of Tofacitinib on Reducing Pain in Patients with Rheumatoid Arthritis, Psoriatic Arthritis and Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/effect-of-tofacitinib-on-reducing-pain-in-patients-with-rheumatoid-arthritis-psoriatic-arthritis-and-ankylosing-spondylitis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-tofacitinib-on-reducing-pain-in-patients-with-rheumatoid-arthritis-psoriatic-arthritis-and-ankylosing-spondylitis/