Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Axial spondyloarthritis (axSpA) is characterized by inflammation of the sacroiliac joints (SIJ) and the spine. Secukinumab (SEC) treatment was clinically efficacious and reduced SIJ bone marrow edema as detected by magnetic resonance imaging (MRI) in patients with non-radiographic (nr)-axSpA through 52 weeks in PREVENT (NCT02696031) study.1 Here, we report radiographic progression and the course of inflammation as assessed by X-ray and MRI of SIJ and spine, over 2 years in the study.
Methods: Study design, key endpoints have been reported.1 In total, 555 patients were randomized (1:1:1) to receive SEC 150mg with (LD) or without loading (NL) doses, or placebo (PBO). Switch to open-label (OL) SEC or standard of care (SoC) was permitted after Week 20. All patients (except those who switched to SoC) received OL SEC from Week 52. Radiographs of the spine and SIJ were collected at baseline (BL) and Week 104; MR images of the spine and SIJ were collected at BL, Weeks 16, 52, and 104. Spinal radiographs were scored using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) and SIJ radiographs according to modified New York criteria (mNYC). Spinal MR images were assessed for signs of inflammation with the Berlin score. SIJ bone marrow edema was assessed according to the Berlin Active Inflammatory Lesions Scoring. All images were evaluated in blinded fashion independently by two central readers. All data are reported from the Week 104 reading session and are presented as observed.
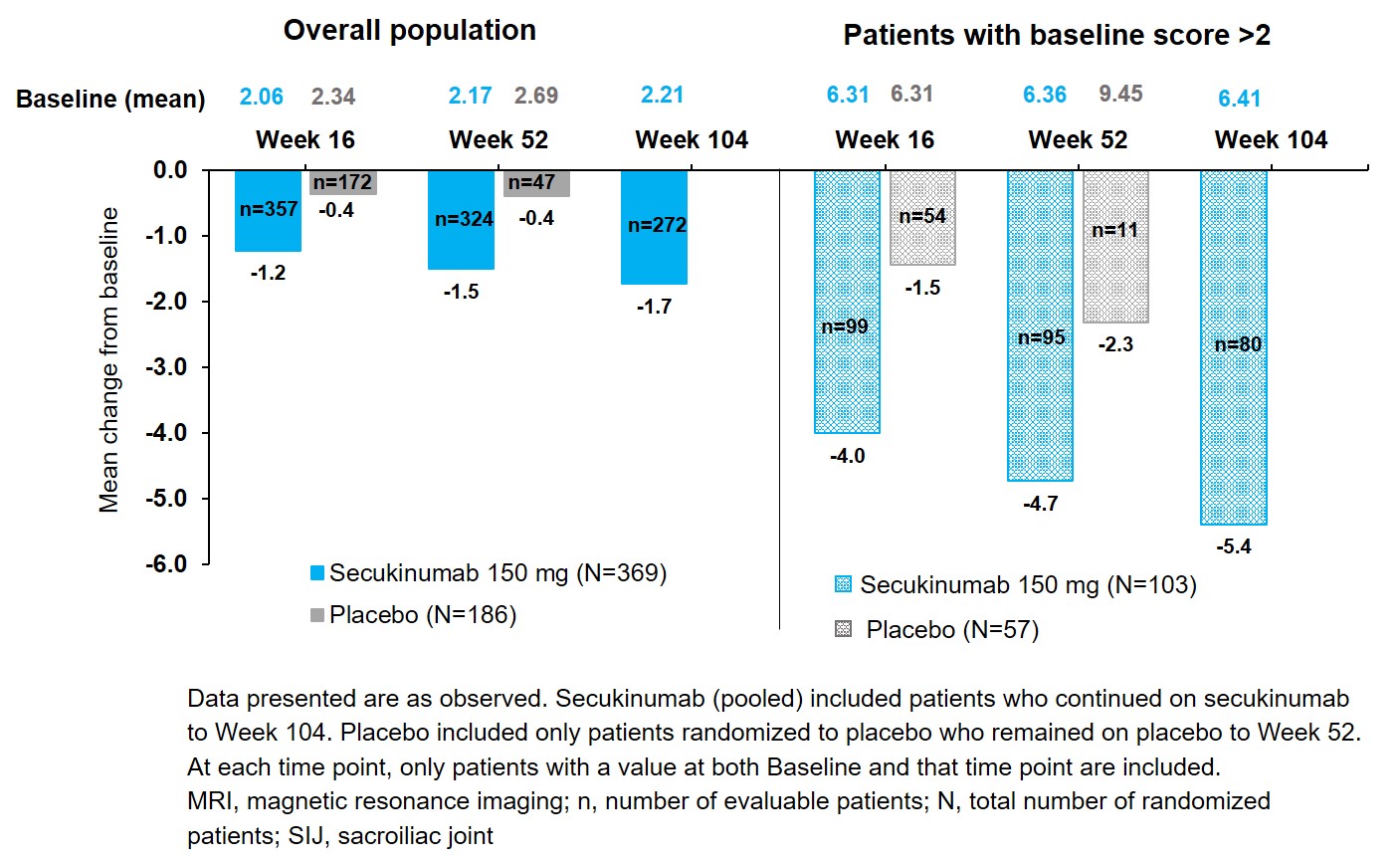
Results: The vast majority (98%) of patients treated with SEC 150mg (pooled LD and NL) showed no structural progression, defined as change in total mSASSS score ≤ smallest detectable change (SDC) of 0.760 (80% agreement level) over 2 years. At BL, 62 patients (43 in SEC 150mg, 19 in PBO) presented with at least one syndesmophyte (at least one vertebral unit scored by at least 1 reader). Among these patients, 9 in the SEC 150mg (20.9%) and 7 in the PBO (36.8%) groups had developed at least one new syndesmophyte by Week 104. Among 237 (SEC 150mg) and 117 (PBO) patients without syndesmophytes at BL, only 4 patients on SEC 150mg (1.7%) and 4 patients on PBO (3.4%) developed at least one new syndesmophyte by Week 104. SIJ radiographs showed that 88% (SEC 150mg) and 86% (PBO) patients had no progression in SIJ [defined as change ≤ SDC (0.458) in total mNYC score] by Week 104. No patient had an increase in that score of 2 or more. Spinal inflammation on MRI (Berlin score) was low at BL: mean 0.82 (SEC 150mg) and 1.07 (PBO) with no change at Week 104: 0.56 (SEC 150mg). SEC reduced SIJ bone marrow edema score versus PBO at Week 16 and Week 52 with sustained reduction through Week 104 in the overall patient population, with greater reduction in patients with BL score > 2 (Figure).
Conclusion: Secukinumab reduced SIJ inflammation (SIJ bone marrow edema) in patients with active nr-axSpA. The majority of patients initially randomized to secukinumab or placebo showed no radiographic progression through 2 years.
References:
1. Deodhar A, et al. Arthritis Rheumatol. 2021; 73:110–20.
To cite this abstract in AMA style:
Braun J, Blanco R, Marzo-Ortega H, Gensler L, Van den Bosch F, Hall S, Kameda H, Poddubnyy D, van de Sande M, van der Heijde D, Zhuang T, Stefanska A, Readie A, Richards H, Deodhar A. Effect of Secukinumab on Radiographic Progression and Inflammation in Sacroiliac Joints and Spine in Patients with Non-radiographic Axial Spondyloarthritis: 2-year Imaging Outcomes from a Phase III Randomized Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-secukinumab-on-radiographic-progression-and-inflammation-in-sacroiliac-joints-and-spine-in-patients-with-non-radiographic-axial-spondyloarthritis-2-year-imaging-outcomes-from-a-phase-iii-ra/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-secukinumab-on-radiographic-progression-and-inflammation-in-sacroiliac-joints-and-spine-in-patients-with-non-radiographic-axial-spondyloarthritis-2-year-imaging-outcomes-from-a-phase-iii-ra/