Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
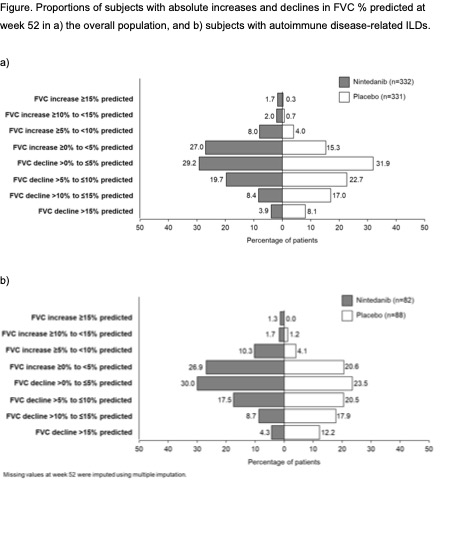
Background/Purpose: In the INBUILD trial in subjects with progressive fibrosing interstitial lung diseases (ILDs) other than idiopathic pulmonary fibrosis (IPF), nintedanib reduced the rate of decline in FVC (mL/year) over 52 weeks by 57% versus placebo. We assessed the effect of nintedanib on categorical changes in FVC % predicted over 52 weeks.
Methods: Subjects in the INBUILD trial had diffuse fibrosing ILD (reticular abnormality with traction bronchiectasis, with or without honeycombing) of >10% extent on HRCT, FVC ≥45% predicted, DLco ≥30%–< 80% predicted, and met criteria for progression of ILD within the 24 months before screening, despite management deemed appropriate in clinical practice. In post-hoc analyses, we assessed the proportions of subjects with categorical absolute increases or declines in FVC % predicted at week 52. Missing values at week 52 were imputed using multiple imputation. Analyses were descriptive.
Results: Overall, 332 subjects were treated with nintedanib and 331 received placebo. At baseline, mean (SD) FVC was 68.7 (16.0) % predicted in the nintedanib group and 69.3 (15.2) % predicted in the placebo group. In the nintedanib and placebo groups, respectively, 19.7% and 22.7% of subjects had absolute declines in FVC of >5% to ≤10% predicted and 12.3% and 25.1% had absolute declines in FVC >10% predicted, while 11.7% and 5.0% of subjects had absolute increases in FVC of ≥5% predicted (Figure). Similar findings were observed in the subgroup of 170 patients with autoimmune disease related ILDs (Figure).
Conclusion: In the overall population of the INBUILD trial and in the subgroup of subjects with autoimmune disease-related ILDs, the proportions of subjects with clinically relevant declines in FVC over 52 weeks were lower in the nintedanib group than in the placebo group. These results provide further support for the benefit of nintedanib on slowing the progression of ILD in subjects with progressive fibrosing ILD other than IPF.
To cite this abstract in AMA style:
Maher T, Cerri S, Hallowell R, Koschel D, Pope J, Tolle L, Mueller H, Rohr K, Inoue Y. Effect of Nintedanib on Categorical Changes in FVC in Patients with Progressive Fibrosing ILDs: Further Analyses of the INBUILD Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-nintedanib-on-categorical-changes-in-fvc-in-patients-with-progressive-fibrosing-ilds-further-analyses-of-the-inbuild-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-nintedanib-on-categorical-changes-in-fvc-in-patients-with-progressive-fibrosing-ilds-further-analyses-of-the-inbuild-trial/