Session Information
Date: Tuesday, November 9, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III (1836–1861)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
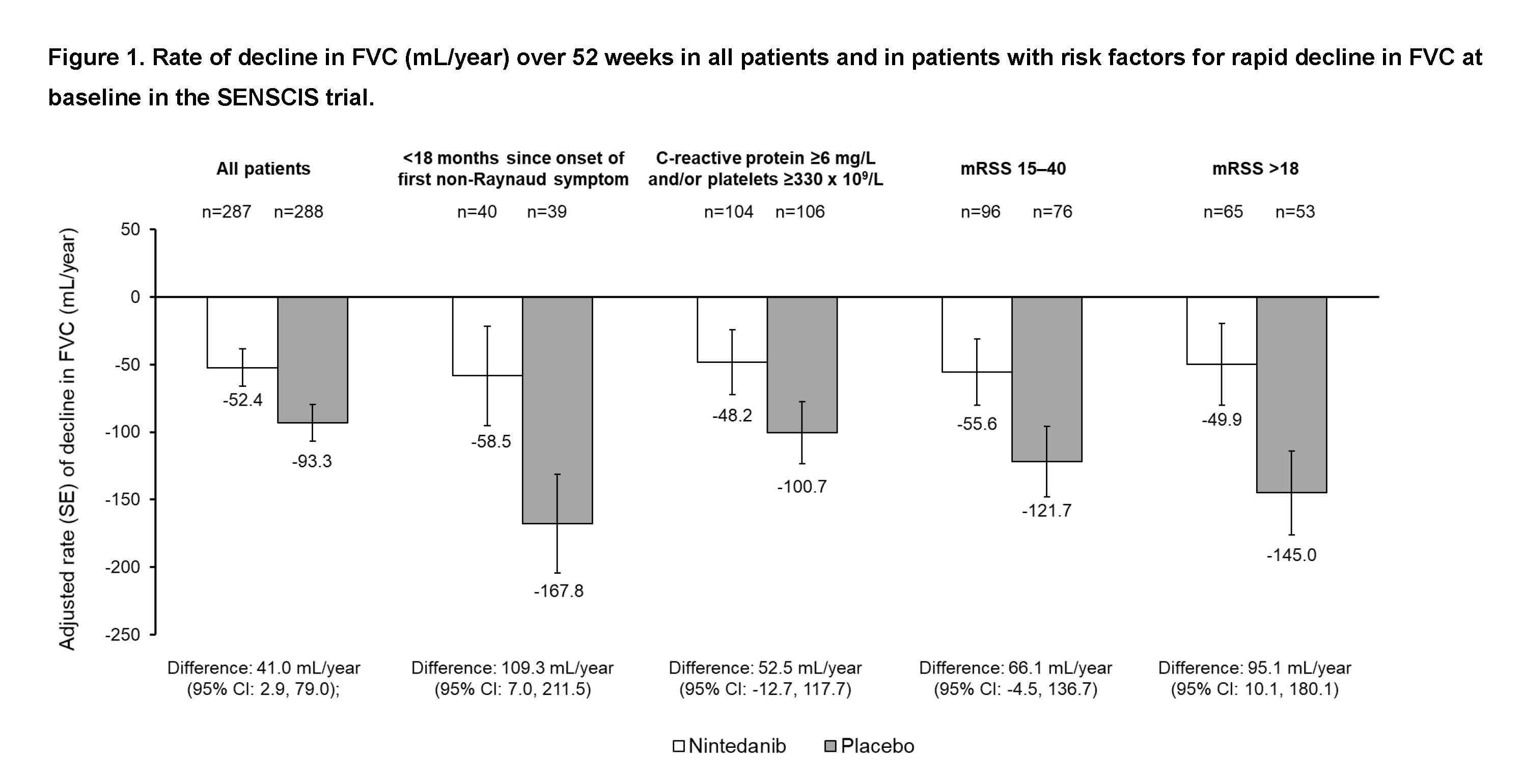
Background/Purpose: In the SENSCIS trial conducted in a population of subjects with systemic sclerosis-associated interstitial lung disease (SSc-ILD), with a mean time since onset of first non-Raynaud symptom of 3.5 years and 52% with diffuse cutaneous SSc (dcSSc), nintedanib reduced the rate of decline in FVC (mL/year) over 52 weeks by 44% versus placebo. Risk factors for a rapid decline in FVC in patients with SSc include early SSc, elevated inflammatory markers, significant skin involvement, and dcSSc. Patients with SSc with these risk factors for rapid progression of ILD are typically given immunosuppressants but not nintedanib. We analyzed the rate of decline in FVC and the effect of nintedanib on FVC decline in subjects with these risk factors in the SENSCIS trial.
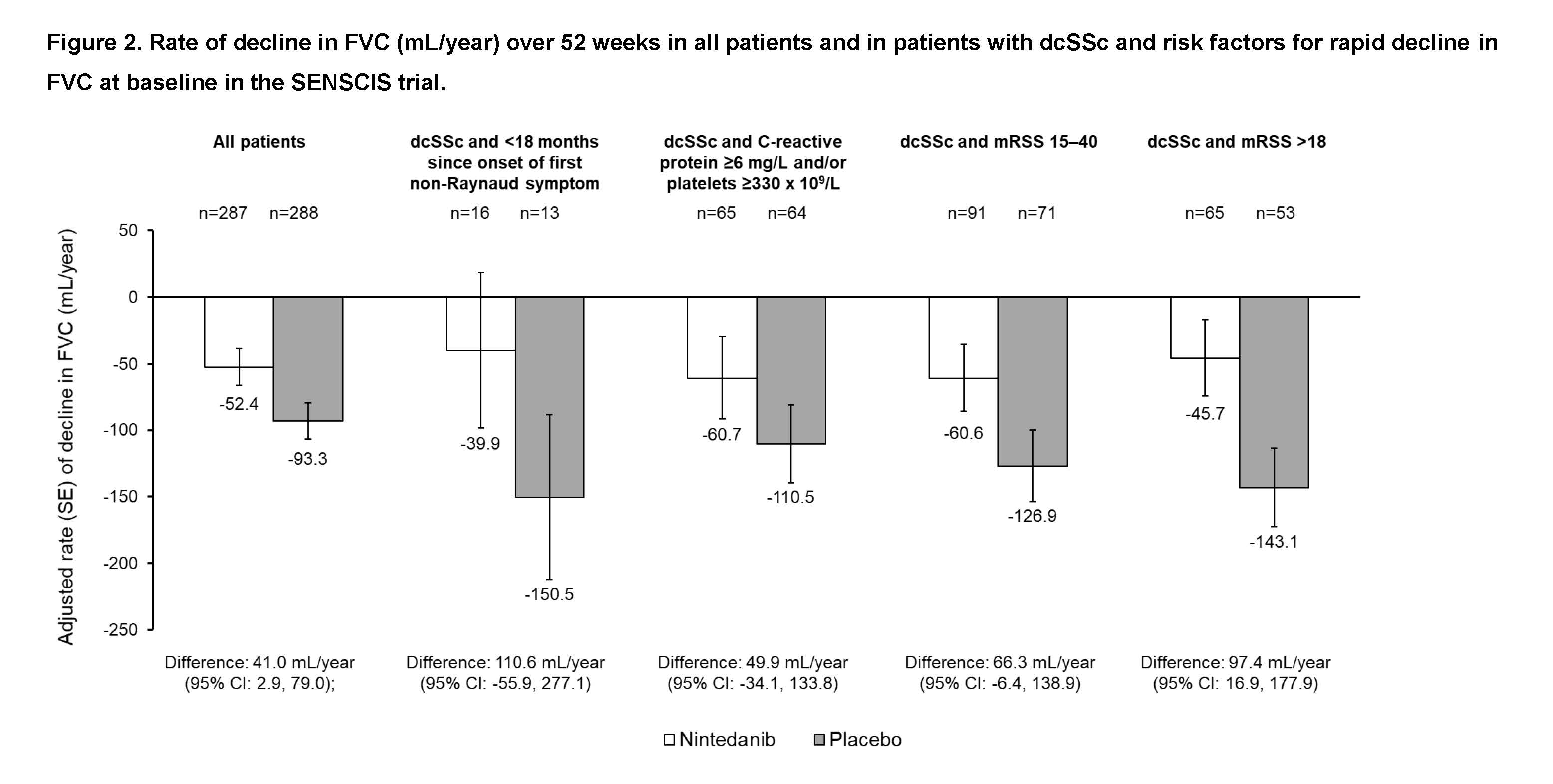
Methods: In post-hoc analyses of data from the SENSCIS trial, we analyzed the rate of decline in FVC (mL/year) over 52 weeks in all subjects and in those with early SSc (< 18 months since onset of first non-Raynaud symptom), elevated inflammatory markers (C-reactive protein ≥6 mg/L and/or platelets ≥330 x 109/L), or significant skin fibrosis using two approaches (modified Rodnan skin score [mRSS] 15-40 or mRSS >18) at baseline. We also analyzed the rate of decline in FVC over 52 weeks in subjects with one of these risk factors and dcSSc.
Results: Of 575 subjects analyzed, 79 (13.7%) had < 18 months since onset of first non-Raynaud symptom, 210 (36.5%) had elevated inflammatory markers, 172 (29.9%) had mRSS 15-40 and 118 (20.5%) had mRSS >18. Of 299 subjects with dcSSc, 29 (9.7%) had < 18 months since onset of first non-Raynaud symptom, 129 (43.1%) had elevated inflammatory markers, 162 (54.2%) had mRSS 15-40 and 118 (39.5%) had mRSS >18. In the placebo group, the rate of decline in FVC over 52 weeks was numerically greater in subjects with these risk factors for rapid decline in FVC compared with all subjects. Across the subgroups, the rate of decline in FVC was numerically lower in subjects treated with nintedanib than placebo (Figures).
Conclusion: The SENSCIS trial included a broad range of subjects with a fibrotic ILD complicating SSc, including those with risk factors for a rapid decline in FVC. In the placebo group, subjects with these risk factors had a more rapid decline in FVC over 52 weeks compared with the overall trial population. By targeting fibrosis with nintedanib, the rate of decline in FVC in patients with risk factors for FVC decline was reduced in patients treated with nintedanib than placebo.
To cite this abstract in AMA style:
Khanna D, Maher T, Volkmann E, Allanore Y, Smith V, Assassi S, Kreuter M, Hoffmann-Vold A, Kuwana M, Stock C, Alves M, Sambevski S, Denton C. Effect of Nintedanib in Patients with Systemic Sclerosis-associated Interstitial Lung Disease and Risk Factors for Rapid Decline in Forced Vital Capacity: Further Analyses of the SENSCIS Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-nintedanib-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-and-risk-factors-for-rapid-decline-in-forced-vital-capacity-further-analyses-of-the-senscis-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-nintedanib-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-and-risk-factors-for-rapid-decline-in-forced-vital-capacity-further-analyses-of-the-senscis-trial/