Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Up to 87% of patients with anti-neutrophil cytoplasmic antibody (ANCA)- associated vasculitis (AAV) have ear, nose and throat (ENT) involvement, which can lead to permanent damage and negatively impacts quality of life. Unfortunately guidelines on the optimal management of ENT symptoms are currently lacking and only few studies, mostly with small patient numbers, evaluated the effect of systemic therapies on ENT disease activity. The aim of this study was therefore to compare the impact of induction therapies on ENT symptoms in patients with AAV.
Methods: In this retrospective study, AAV patients with ENT involvement treated at one of the two Dutch centers between January 2010 and April 2020 were included. Clinical, histological and laboratory data were collected from electronic patient records. ENT involvement was defined as 1) at least one ENT symptom according to the Birmingham vasculitis activity score (BVAS) and/or 2) presence of saddle nose deformity. Regression analyses were used to identify associations between the induction agents cyclophosphamide (CYC) and rituximab (RTX) and disease activity while correcting for confounders (follow-up time, age, sex, renal and pulmonary involvement). Some patients (N=41) had a period of treatment with CYC and a treatment period with RTX over the follow-up period. This dependency was taken into account using Generalized Estimating Equation.
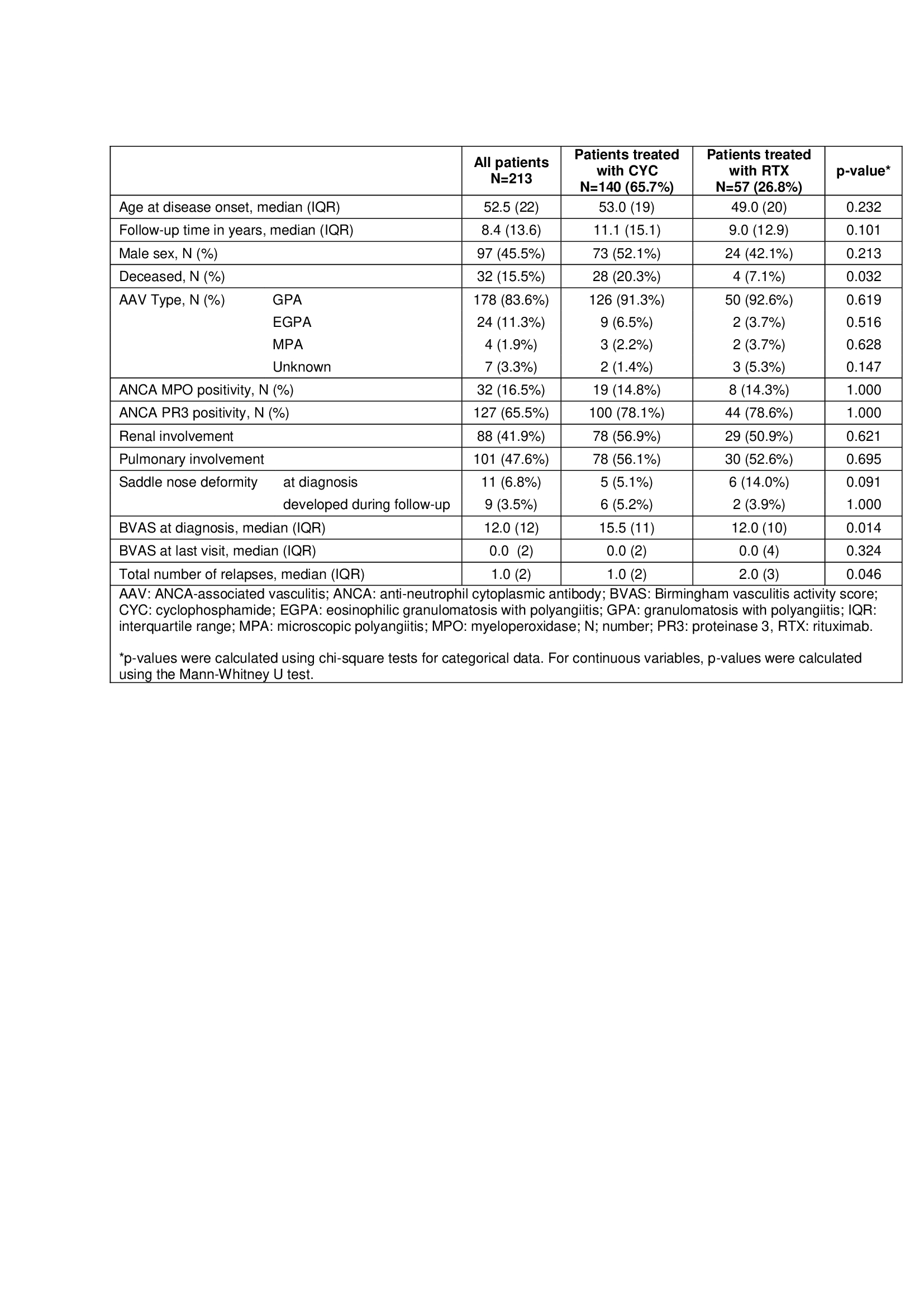
Results: In our centers, 213 (67.8%) of 320 AAV patients had ENT involvement. In these 213 patients, median age at disease onset was 52.5 years (IQR 22) and 45.5% was male (N=97). Median BVAS was 12 (IQR 12) at diagnosis and 0 (IQR 2) at last visit. Relapse rate was 0.06 per year (IQR 0.17). At relapse 49.7% (N=78) had ENT involvement and 29.0% (N=60) had ENT activity at last visit (Table 1).
Male patients had a lower risk of ENT activity at last follow-up (OR 0.31 (CI 0.16-0.62), p=0.001) compared to females, as did patients with a lower age at disease onset (OR 0.98 (CI 0.95-0.99), p=0.020). Renal and pulmonary involvement, biopsy results, and ANCA titer, ESR and CRP at diagnosis were not significantly associated with ENT activity at last visit.
In total 140 (65.7%) patients were treated with CYC and 57 (26.8%) with RTX. No statistically significant difference in ENT activity at last visit was observed between patients treated with oral or intravenous CYC compared to RTX (adjusted OR 0.61 (95% CI 0.34-1.09), p=0.093). Furthermore, there was no significant difference in the number of patients with at least one ENT relapse between the RTX and CYC group (adjusted OR 0.61 (CI 0.30-1.28), p=0.192). Eight (3.8%) patients received methotrexate as induction therapy. In these patients median age at disease onset was 45.5 years (IQR 30) and 75.0% (N=6) was female. These eight patients had a median relapse rate of < 0.01 per year (IQR 0.16) and three (37.5%) patients had ENT activity at last follow-up.
Conclusion: We found no significant difference in ENT activity at last visit or history of ENT relapse between CYC and RTX treated patients. Importantly, persistent ENT symptoms during flares were observed in a considerable number of patients, which emphasizes the need for further research on optimal management of ENT symptoms in AAV.
To cite this abstract in AMA style:
Krol R, Schaap C, Welsing P, Klaasen R, Remmelts H, Hagen C, van Reekum F, Heijstek M, Spierings J. Effect of Induction Therapies on Ear, Nose and Throat Involvement in Anti-neutrophil Cytoplasmic Antibody Associated Vasculitis: Results from a Multi-center Cohort Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-induction-therapies-on-ear-nose-and-throat-involvement-in-anti-neutrophil-cytoplasmic-antibody-associated-vasculitis-results-from-a-multi-center-cohort-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-induction-therapies-on-ear-nose-and-throat-involvement-in-anti-neutrophil-cytoplasmic-antibody-associated-vasculitis-results-from-a-multi-center-cohort-study/