Session Information
Date: Tuesday, November 9, 2021
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II (1565–1583)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Observational studies have reported that serum urate associates with development and progression of kidney disease. However, it is uncertain whether elevated serum urate directly influences kidney function, with recent Mendelian randomization studies and clinical trials of urate-lowering therapy in CKD suggesting that elevated urate does not influence kidney disease. Inosine is a purine nucleoside that increases extra-renal urate production and thus, serum urate concentrations. We analyzed data from a 6-month randomized placebo-controlled trial of inosine supplementation to determine whether moderate hyperuricemia induced by inosine affects kidney function, as assessed by serum creatinine.
Methods: One hundred and twenty post-menopausal women were recruited into a six-month randomized, double-blind, placebo-controlled trial of inosine supplementation for bone health (Arthritis Rheumatology 2021, PMID: 33586367). Exclusion criteria included gout, kidney stones, eGFR≤60 ml/min/1.73m2, and urine pH ≤5.0. Participants were randomized 1:1 to inosine supplements (doses adjusted to maintain serum urate below 8mg/dL), or placebo. Serum creatinine, serum urate, and fractional excretion of uric acid (FEUA) were pre-specified secondary endpoints. Data were analyzed on an intention to treat basis, using a mixed models approach to repeated measures, with false detection rate protected pairwise comparisons at each time point. For analysis of covariance of change from baseline (ANCOVA), baseline values were included in the models.
Results: Administration of inosine supplements led to a significant increase in serum urate and FEUA over the study period (Figure 1A and 1B respectively). The maximum difference between groups in both serum urate and FEUA was observed at week 6; at this time point, the mean (SD) serum urate was 7.3 (1.5) mg/dL in the inosine group, and 4.7 (1.0) mg/dL in the placebo group (P< 0.0001), and the mean (SD) FEUA was 10.1 (3.2)% in the inosine group and 7.7 (2.4)% in the placebo group (P< 0.0001). There was no between-group difference in serum creatinine values at any time point over the 26 week period (P>0.62 Figure 1C). Analysis of the change in serum creatinine from baseline showed a maximal difference between groups at week 13 (with mean (95% CI) difference of +0.029 (-0.005, +0.055) mg/dL, P=0.059, Figure 1D). At all other time points, there was no between-group difference in the change in serum creatinine.
Conclusion: Elevated serum urate due to increased extra-renal urate production does not lead to kidney impairment over a six month period.
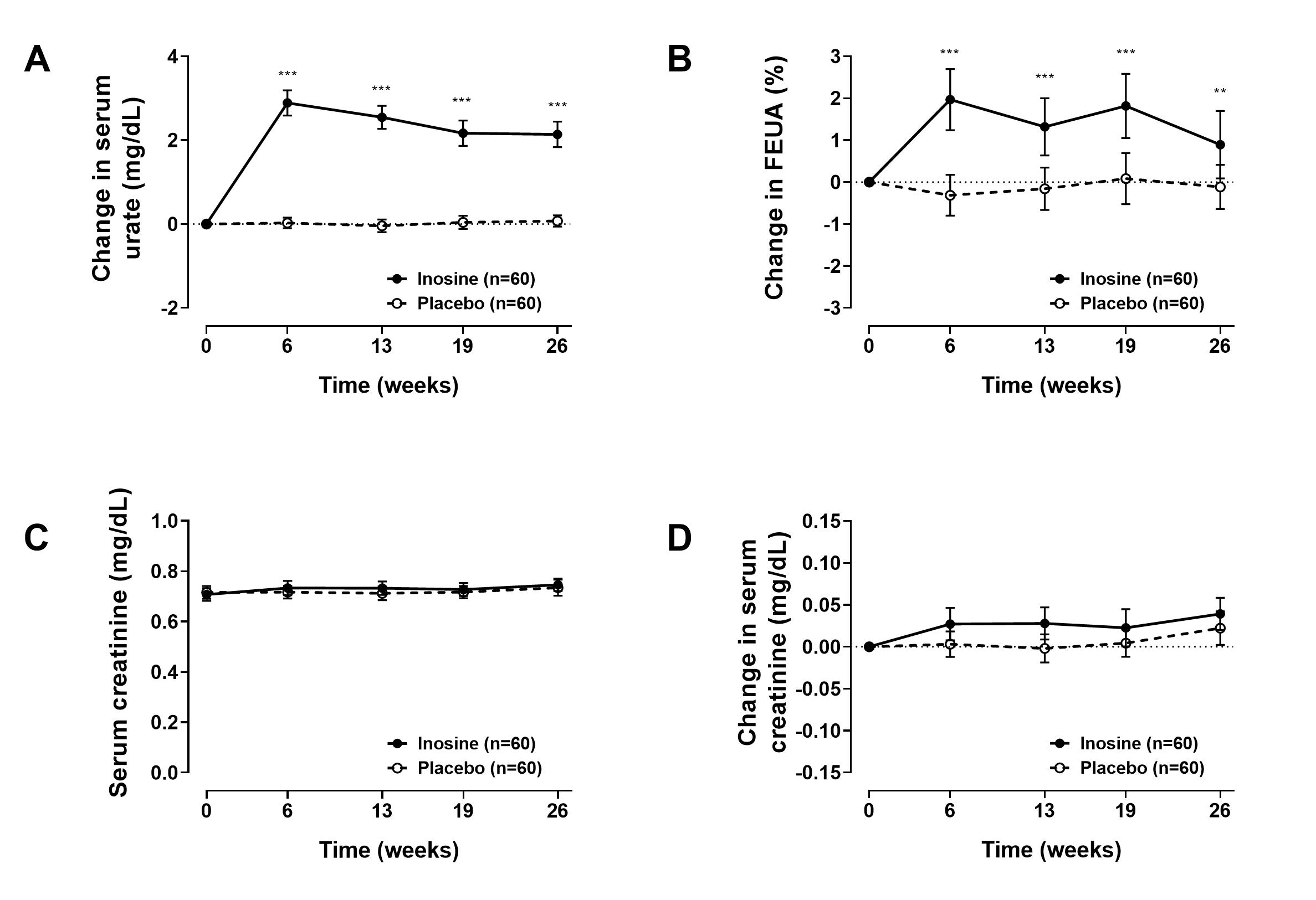 Figure 1. Study endpoints. A. Change in serum urate; B. Change in FEUA; C. Serum creatinine values; D. Change in serum creatinine; Data re presented as mean (95% CI). **false detection rate P < 0.01, ***false detection rate P < 0.001.
Figure 1. Study endpoints. A. Change in serum urate; B. Change in FEUA; C. Serum creatinine values; D. Change in serum creatinine; Data re presented as mean (95% CI). **false detection rate P < 0.01, ***false detection rate P < 0.001.
To cite this abstract in AMA style:
Dalbeth N, Mihov B, Stewart A, Gamble G, Merriman T, Mount D, Stamp L, Reid I, Horne A. Effect of Elevated Serum Urate on Kidney Function: Analysis of a Randomized Controlled Trial of Inosine Supplementation [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-elevated-serum-urate-on-kidney-function-analysis-of-a-randomized-controlled-trial-of-inosine-supplementation/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-elevated-serum-urate-on-kidney-function-analysis-of-a-randomized-controlled-trial-of-inosine-supplementation/
