Session Information
Date: Monday, November 9, 2020
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Giant cell arteritis (GCA) is a form of large vessel vasculitis that requires treatment with high-dose, long-term glucocorticoids (GC). Weight gain, among other side-effects of GC therapy, is a major concern for patients and is associated with increased morbidity and mortality. Prior studies have shown varying results regarding the independent association of GC dose and weight gain. Weight changes in GCA patients being treated with GC or in GCA patients receiving tocilizumab are poorly understood.
Methods: We analyzed GCA patients enrolled in the GiACTA (Tocilizumab in Giant Cell Arteritis) trial. Cumulative GC dose and weight were obtained at weeks 0, 12, 24, and 52. We used multivariable mixed-effects modeling to examine the effects of age, gender, cumulative GC dose, baseline GC exposure, disease status (newly-diagnosed versus relapsing), randomization to tocilizumab, and flares over 52 weeks on changes in weight. We used Student’s unpaired t-test to evaluate weight change from 0 to 52 weeks and the Wilcoxon rank test to evaluate the cumulative prednisone from 0 to 52 weeks.
Results: 250 patients were included; 187 (75%) were female and the mean age ± SD was 69 ± 8 years. The mean baseline weight ± SD was 70.8 ± 14.9 kg, and the mean ± SD weight gain among all subjects was 3.2 ± 5.4 kg. Cumulative prednisone dose at 52 weeks was associated with weight change over this time (0.63kg per 1000mg of prednisone; p< 0.001) (Table 1). Furthermore, relapsing disease at baseline and flares over 52 weeks were significantly associated with less weight gain. Among patients who received tocilizumab, those who met the primary endpoint of sustained, glucocorticoid-free remission at 52 weeks received less prednisone than did those who failed (median 1356 vs. 3017mg; p< 0.0001), but these subgroups experienced similar changes in weight (mean 3.0 vs. 4.0kg; p=0.31) (Table 2). Among patients who received prednisone alone, those who met the primary endpoint received less prednisone (median 2150 vs. 4284mg; p< 0.0001), yet those patients experienced numerically higher gains in weight compared with those who did not meet the primary endpoint (mean 3.7 vs. 2.8kg; p=0.59).
Conclusion: GC use and weight gain are linked tightly in the minds of providers and patients, but the relationship is actually complex. We found that cumulative GC exposure over 52 weeks is significantly associated with weight gain. However, the degree to which disease activity is controlled also contributes to weight gain, and modest weight gain may be an indicator of effective disease control. Additional research is required to better elucidate this relationship and to understand other determinants of excessive weight gain in this patient population.
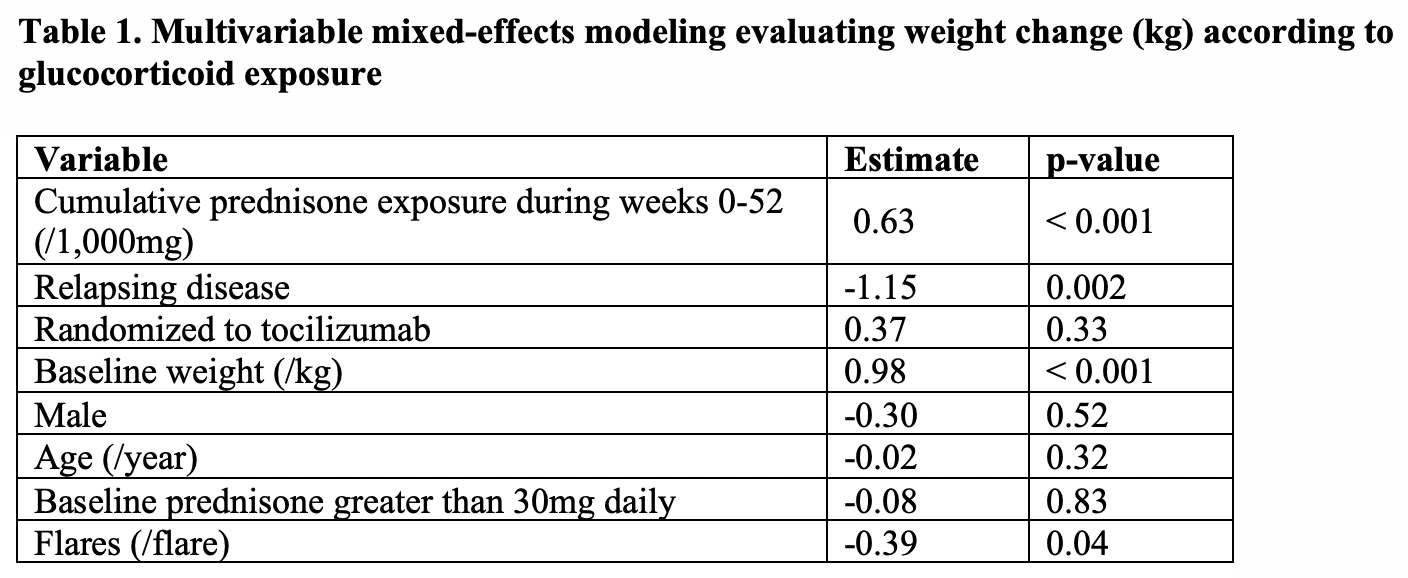 Table 1. Multivariable mixed-effects modeling evaluating weight change (kg) according to glucocorticoid exposure
Table 1. Multivariable mixed-effects modeling evaluating weight change (kg) according to glucocorticoid exposure
 Table 2. Cumulative prednisone exposure and weight change per treatment group, stratified by primary endpoint of sustained, glucocorticoid-free remission at 52 weeks
Table 2. Cumulative prednisone exposure and weight change per treatment group, stratified by primary endpoint of sustained, glucocorticoid-free remission at 52 weeks
To cite this abstract in AMA style:
Serling-Boyd N, Fu X, Zhang Y, Unizony S, Wallace Z, Choi H, Stone J. Effect of Cumulative Glucocorticoid Dose and Inflammation on Weight Change During Treatment of Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effect-of-cumulative-glucocorticoid-dose-and-inflammation-on-weight-change-during-treatment-of-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-cumulative-glucocorticoid-dose-and-inflammation-on-weight-change-during-treatment-of-giant-cell-arteritis/
