Session Information
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment: Spondyloarthritis I
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Axial spondyloarthritis (axSpA ) includes both ankylosing spondylitis (AS) and non-radiographic axSpA (nr-axSpA), as defined by the ASAS criteria.1 RAPID-axSpA (NCT01087762) is the first report of certolizumab pegol (CZP), a PEGylated Fc-free anti-TNF, in axSpA, and the first randomized controlled trial of an anti-TNF to include the entire axSpA population.
Methods:
The ongoing 158 week (wk) RAPID-axSpA trial is double-blind and placebo (PBO) controlled to Wk24, dose-blind to Wk48 and then open label to Wk158. Recruited patients (pts) had adult-onset active axSpA as defined by the ASAS criteria,1 BASDAI ≥4, spinal pain ≥4 on a 10 point scale, CRP > upper limit of normal or sacroillitis on MRI. Pts must have failed ≥1 NSAID. Up to 40% of pts could have experienced secondary failure to 1 previous anti-TNF. The pt population reflected the broad axSpA population, including AS pts meeting the modified New York criteria and nr-axSpA pts who met the ASAS MRI or clinical criteria. Pts were randomized 1:1:1 to PBO, or 400mg CZP at Wk 0, 2 and 4 (loading dose, LD) followed by either 200mg CZP every 2 wks (Q2W) or 400mg CZP every 4 wks (Q4W). Pts receiving PBO who failed to achieve an ASAS20 response at both Wks 14 and 16 were rescued and randomized at Wk16 to receive CZP 200mg Q2W or CZP 400mg Q4W following LD. The primary endpoint was ASAS20 response at Wk12. Non-responder imputation was used for ASAS responses; last observation carried forward was used for BASFI, BASMI and BASDAI.
Results:
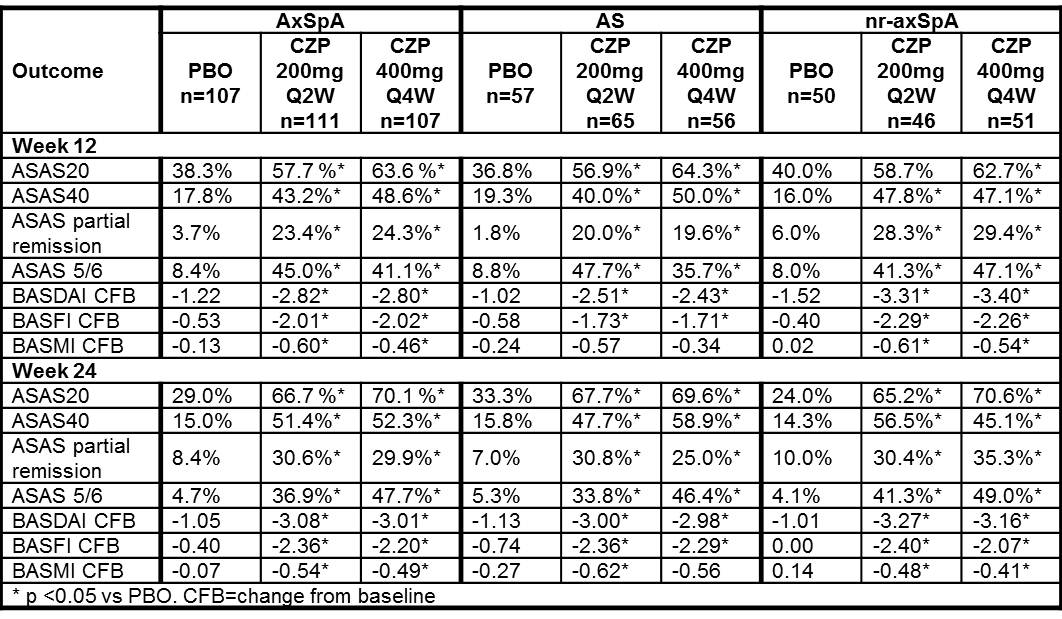
325 pts were randomized. Baseline (BL) characteristics were similar between treatment groups (PBO/CZP 200 mg Q2W/CZP 400 mg Q4W) with the exception of higher CRP concentrations, and greater proportion of anti-TNF experienced pts in the PBO group (25.4/17.4/21.2 mg/L; 24.3%/13.5%/10.3%, respectively). AS pts had longer disease and symptom duration at BL compared to nr-axSpA pts. Clinical improvements in ASAS20 responses at Wk12 were statistically significant in CZP 200 mg Q2W and CZP 400 mg Q4W arms vs PBO (Table) and were observed as early as Wk1 (40.5% and 34.6% vs 14.2%, p<0.001). ASAS40 response and ASAS partial remission rates were also higher in the CZP arms vs PBO. At Wks12 and 24, combined CZP arms showed statistically significant improvements vs PBO in BASDAI, BASFI, and BASMI. Similar improvements were reported with CZP vs PBO in both AS and nr-axSpA pts (Table). Adverse events (AEs) occurred in 70.4% vs 62.6%, serious AEs in 4.7% vs 4.7%, and serious infections in 1.1% vs 0 of CZP (combined dose) pts vs PBO pts, respectively. No deaths, TB or malignancies were reported.
Conclusion:
CZP effectively and rapidly reduced the signs and symptoms of axSpA, including spinal mobility, with no new safety signals observed. Improvements were similar across CZP dosing regimens, and observed in both AS and nr-axSpA pts.
References:
- Rudwaleit M. Ann Rheum Dis. 2009;68(6):770-776
Table: Clinical outcomes at Wk12 and Wk24 for axSpA pts in RAPID-axSpA
Disclosure:
R. B. M. Landewé,
UCP Pharma,
5,
UCB Pharma,
2;
M. Rudwaleit,
UCB Pharma,
5;
D. van der Heijde,
UCB Pharma,
5,
UCB Phama,
2,
UCB Pharma,
8;
M. Dougados,
UCB Pharma ,
2,
UCB Pharma,
5;
W. P. Maksymowych,
UCB Pharma,
5,
UCB Pharma,
2,
UCB Pharma,
8;
J. Braun,
UCB Pharma,
2,
UCB Pharma,
5,
UCB Pharma,
8;
A. A. Deodhar,
UCB Pharma,
2,
UCB Pharma,
5;
C. Stach,
UCB Pharma,
3;
B. Hoepken,
UCB Pharma,
3;
G. Coteur,
UCB Pharma,
3;
D. Kielar,
UCB Pharma,
3,
UCB Pharma,
1;
A. Fichtner,
UCB Pharma,
3;
T. Arledge,
UCB Pharma,
3;
J. Sieper,
UCB Pharma,
5,
UCB Pharma,
8.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-certolizumab-pegol-on-signs-and-symptoms-of-ankylosing-spondylitis-and-non-radiographic-axial-spondyloarthritis-24-week-results-of-a-double-blind-randomized-placebo-controlled-phase-3/