Session Information
Date: Sunday, November 13, 2022
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Serological and cellular biomarkers are used in clinical practice to guide the management of SLE. Increased levels of anti-dsDNA and decreased levels of complement are often associated with active disease, and predictive of disease flares and progression.1,2 The monoclonal antibody BEL (approved for SLE and LN) inhibits B-lymphocyte stimulator and has been shown to improve biomarkers in individual studies.3 This analysis used pooled data from five studies to evaluate the effects of BEL on B-cells and various serological biomarkers (Igs, autoantibodies, complement) in a large population of adult patients (pts) with SLE.
Methods: The Be-SLE integrated, post hoc analysis assessed data from five double-blind, placebo (PBO)-controlled BEL studies: BLISS-76, BLISS-52, BLISS-NEA, BLISS-SC, and EMBRACE. In all studies, pts with active SLE received BEL (10 mg/kg intravenously or 200 mg subcutaneously) or PBO, plus standard therapy. Median percent changes (interquartile range [IQR]) from baseline over 52 weeks (Wks) were reported for B-cells, Igs, autoantibodies and complement. Wilcoxon rank sum tests (for Igs and autoantibodies) and analysis of covariance (for B-cells and complement) were used to determine statistical differences between BEL- and PBO-treated groups.
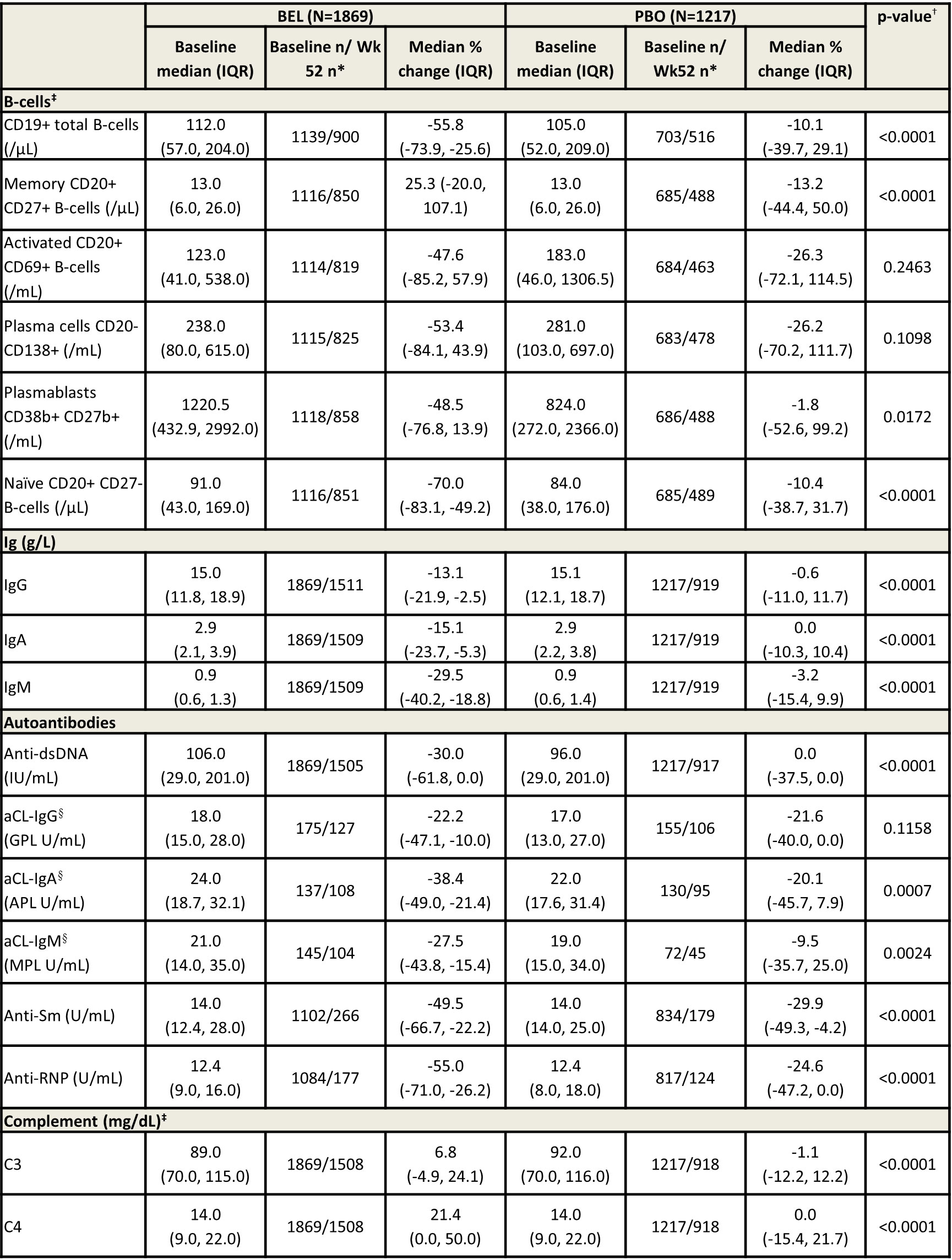
Results: In total, 1869 and 1217 pts received BEL and PBO, respectively. Most were female (BEL, 95%; PBO, 94%) and of Asian, White, or Black African ancestry (BEL, 37%, 32%, and 22%; PBO, 33%, 36%, and 19%, respectively), with a mean (SD) age of 36.7 (11.4) and 37.4 (12.0) among BEL- and PBO-treated pts. Levels of B-cells, Igs, autoantibodies, and complement generally showed larger median (IQR) percent changes among pts receiving BEL versus PBO (Table). For example, CD19+ total B-cells (BEL, -55.8% [-73.9, -25.6]; PBO, -10.1% [-39.7, 29.1]), naïve CD20+ CD27- B-cells (BEL, -70.0% [-83.1, -49.2]; PBO -10.4% [-38.7, 31.7]) and IgM (BEL, -29.5% [-40.2, -18.8]; PBO, -3.2% [-15.4, 9.9]) had significantly greater reductions with BEL than PBO at Wk 52 (all p< 0.0001). Levels of anti-dsDNA (BEL, -30.0% [-61.8, 0.0]; PBO, 0.0% [-37.5, 0.0]), anti-Sm (BEL, -49.5% [-66.7, -22.2]; PBO, -29.9% [-49.3, -4.2]) and anti-RNP (BEL, -55.0% [-71.0, -26.2]; PBO, -24.6% [-47.2, 0.0]) had significantly greater reductions with BEL versus PBO at Wk 52 (all p< 0.0001). Pts receiving BEL also showed a significant increase in levels of complement versus PBO (Table). Changes in B-cells and these serological biomarkers were observed as early as Wk 8 (data not shown).
Conclusion: BEL treatment showed significant improvements in serological biomarkers (including reductions in autoantibodies and increased complement) and B-cell populations versus PBO. Improvements in autoantibodies were observed by Wk 8 and continued to improve over 52 weeks. By combining multiple studies, this analysis provides insights using a larger dataset than would otherwise be available and confirms the benefits of BEL on biomarkers in a large, pooled population.
Funding: GSK
References
1Capecchi R, et al. Rheumatology (Oxford). 2020;59:v12–v18
2Bertsias G, et al. EULAR Textbook on Rheumatic Diseases. 2012;5:476–505
3Levy RA, et al. Lupus. 2021;30:1705–21
*Patients with a baseline value of zero were excluded from analysis at Wk 52; †a p-value of <0.05 was considered significant; ‡covariates for the ANCOVA were treatment group, study, baseline B-cell value or baseline complement value, and baseline SELENA-SLEDAI score; §among patients who were positive at baseline.
ANCOVA, analysis of covariance; mITT, modified intent-to-treat; SELENA, Safety of Estrogens in Systemic Lupus Erythematosus National Assessment
To cite this abstract in AMA style:
Knight J, Chatham W, Henning C, Harris J, Van Maurik A, Levy R, Pisetsky D. Effect of Belimumab (BEL) on B-cells and Serological Biomarkers for SLE: Results of the Large Integrated Analysis BEL Summary of Lupus Efficacy (Be-SLE) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effect-of-belimumab-bel-on-b-cells-and-serological-biomarkers-for-sle-results-of-the-large-integrated-analysis-bel-summary-of-lupus-efficacy-be-sle/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-belimumab-bel-on-b-cells-and-serological-biomarkers-for-sle-results-of-the-large-integrated-analysis-bel-summary-of-lupus-efficacy-be-sle/