Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Entheseal bone changes occur in a substantial proportion of psoriasis patients [1] and are associated with an increased risk of developing psoriatic arthritis (PsA) [2].Herein, we aimed to investigate the effect of phosphodiesterase-4 inhibition by apremilast on entheseal bone changes in psoriasis patients with a high-risk profile for developing PsA.
Methods: Epos (Early PsA on treatment strategy) is a prospective, single-arm, interventional, open-label, single-center phase 4 trial (EUDRACT 2018-000335-27) in psoriasis patients with an increased risk of transition to psoriatic arthritis (PsA). Participants had to have (i) mild to moderate psoriasis, (ii) arthralgia and (iii) subclinical inflammatory changes assessed by hand MRI and/or high-resolution CT. Patients with any present or past signs of PsA (arthritis, dactylitis, enthesitis, axial involvement) as well as previous treatment with biological or targeted-synthetic DMARDs were not allowed to participate. Participants received apremilast 30 mg twice daily over 24 weeks. To assess the effect of apremilast on entheseal bone changes high-resolution CT scans of the entheseal areas of metacarpal finger joints were conducted at baseline and week 24. At the entheseal areas, peripheral volumetric bone mineral density (vBMD), bone microstructure and structural entheseal lesions were assessed. Clinical response and safety were monitored.
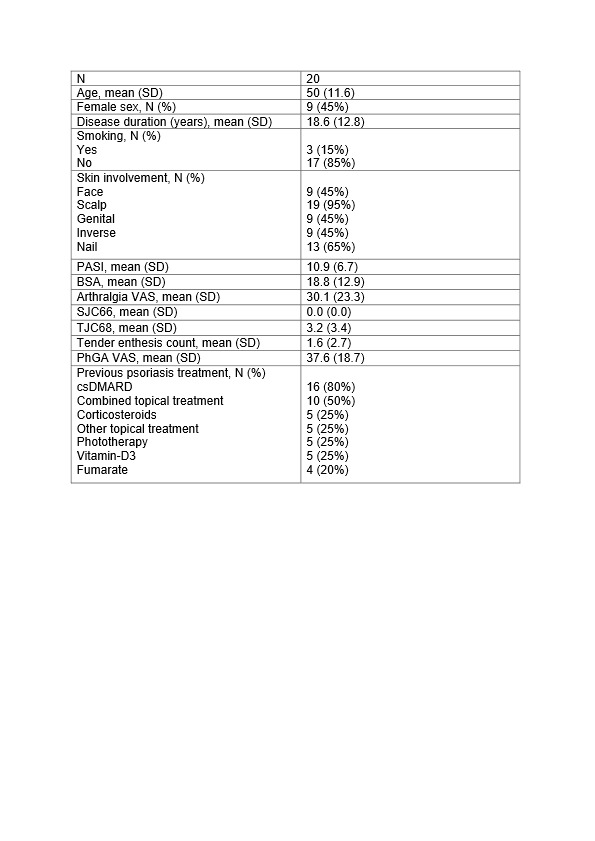
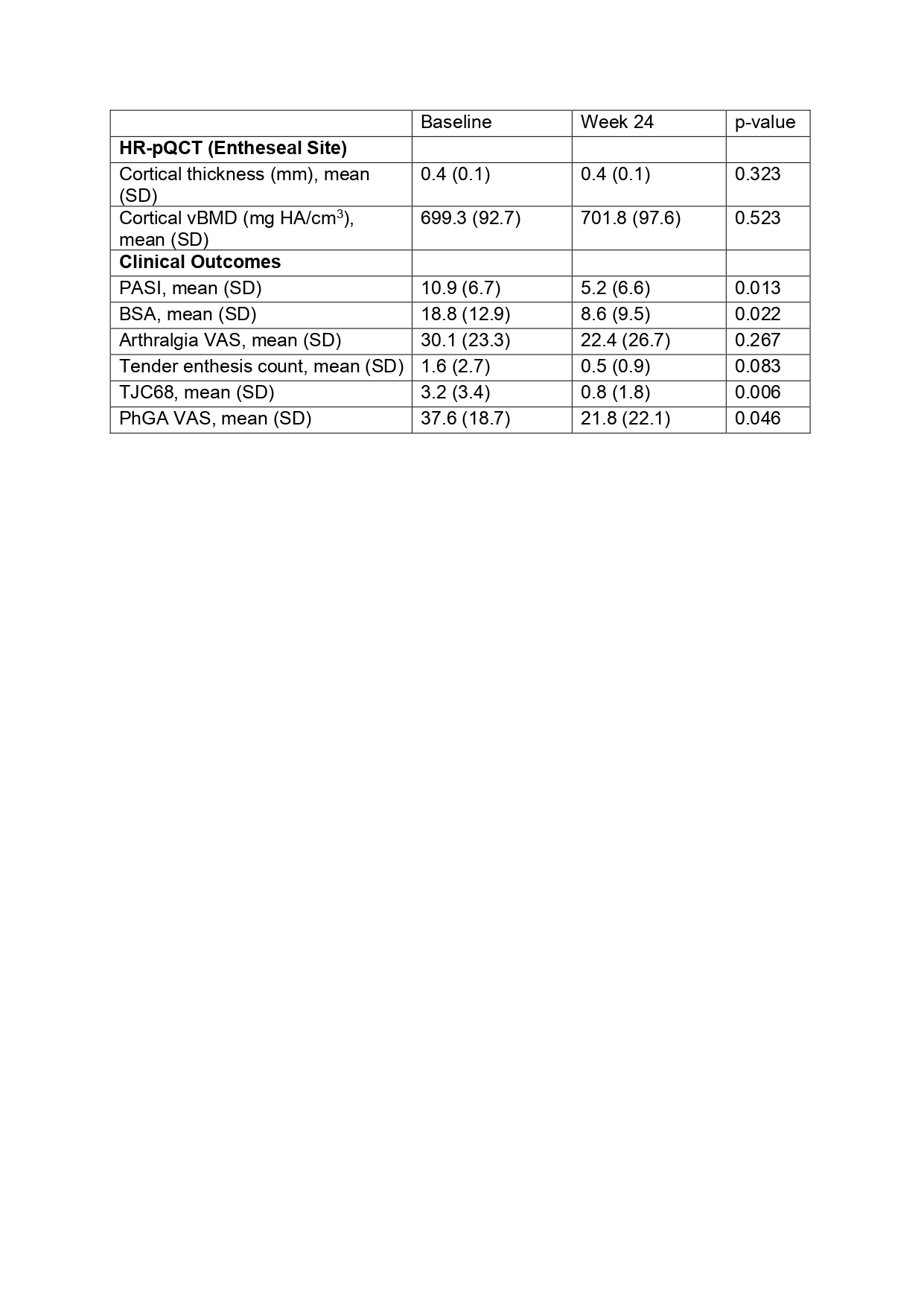
Results: 20 patients (50 [SD 11.6] years; 9 women) with a duration of skin psoriasis of 18.6 [SD 12.8] years were included (table 1). At week 24, skin disease significantly improved (Psoriasis Area and Severity Index: from 10.9±6.7 at baseline to 5.2±6.6 at week 24) as well as arthralgia (VAS: from 30.1±23.3 at baseline to 22.4±26.7 at week 24) (table 2). Tender joint count 68 and tender enthesis count also declined over 24 weeks of treatment (3.2±3.4 at baseline vs. 0.8±1.8 at week 24 and 1.6±2.7 at baseline vs. 0.5±0.9 at week 24, respectively) (table 2). No significant progress was found for entheseal cortical vBMD (baseline: 699.3±92.7 mg HA/cm3vs. week 24: 701.8±97.6 mg HA/cm3, p=0.523) and entheseal cortical thickness (0.4±0.1 mm vs. 0.4±0.1 mm, p=0.323) (table 2). Accordingly, no progression was detected in structural entheseal lesions. No new safety signals with apremilast treatment were observed.
Conclusion: Apremilast leads to early disease interception by improving skin disease and arthralgia and halting progression of entheseal bone changes in psoriasis patients at risk for the transition to PsA. Larger studies with longer follow-up periods are needed to validate these findings.
BSA: Body Surface Area; csDMARD: conventional synthetic Disease Modifying Antirheumatic Drug; PASI: Psoriasis Area and Severity Index; PhGA: Physician’s Global Assessement of disease activity; SD: Standard deviation; SJC66: Swollen Joint Count 66; TJC68: Tender Joint Count 68; VAS: Visual Analogue Scale.
BSA: Body Surface Area; PASI: HR-pQCT: High-Resolution peripheral Quantitative Computed Tomography; Psoriasis Area and Severity Index; PhGA: Physician’s Global Assessement of disease activity; SD: Standard Deviation; TJC68: Tender Joint Count 68; VAS: Visual Analogue Scale; vBMD: volumetric Bone Mineral Density.
To cite this abstract in AMA style:
Simon D, Minopoulou I, Bayat S, Tascilar K, Yalcin Mutlu M, Fagni F, Schett G, Kleyer A. Effect of Apremilast on Disease Interception in Patients with Psoriasis at Increased Risk of Developing PsA – Results of the Prospective Interventional Epos Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/effect-of-apremilast-on-disease-interception-in-patients-with-psoriasis-at-increased-risk-of-developing-psa-results-of-the-prospective-interventional-epos-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-apremilast-on-disease-interception-in-patients-with-psoriasis-at-increased-risk-of-developing-psa-results-of-the-prospective-interventional-epos-trial/