Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Anti-citrullinated protein antibodies (ACPA) are a
marker of RA, and the presence of the immunoglobulin M (IgM) isotype indicates
an ongoing immune response involving the recruitment of naïve B cells.1
Abatacept (ABA) modulates T-cell co-stimulation and has been shown to impact
ACPA maturation, including seroconversion of IgM, in the AVERT (Assessing
Very Early Rheumatoid arthritis Treatment) trial.2
The objective of this analysis was to assess the efficacy of treatment
with ABA+MTX, ABA monotherapy or MTX alone in patients
(pts) from the AVERT trial based on their anti-cyclic
citrullinated peptide 2 (CCP2; a surrogate for ACPA) IgM serostatus at baseline (BL), and seroconversion (anti-CCP2
IgM positive to negative) through 1 year.
In this post hoc analysis, pt samples were analysed by ELISA to
determine anti-CCP2 IgM serostatus. Efficacy outcomes analyzed by BL anti-CCP2
IgM serostatus included remission rate at 12 mths (CDAI, SDAI, Boolean and
DAS28 [CRP] <2.6-defined remission), and adjusted mean change in DAS28 (CRP)
and HAQ-DI over time (samples taken every 28 days up to Mth 12 and analyzed
with a longitudinal repeated-measures model). Boolean
remission was analyzed in pts who seroconverted.
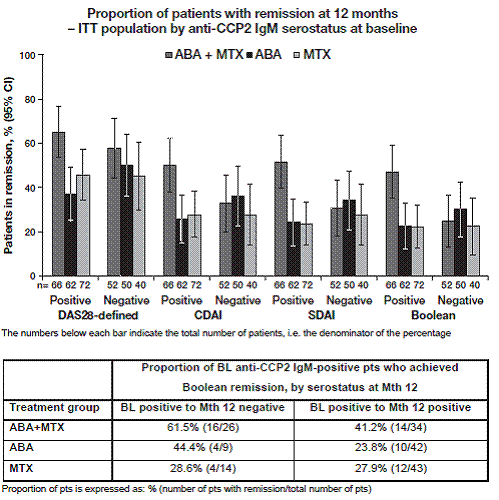
anti-CCP2 IgM positive at BL achieved remission by all indices compared with
pts who were BL IgM negative (Figure). This trend was most clearly observed in
the stringent indices of CDAI, SDAI and Boolean remission, compared with DAS28
(CRP)-defined remission. This trend was not observed in either the ABA
monotherapy or MTX alone arms. Mean improvement in DAS28 (CRP) and HAQ-DI
over time was also greatest in BL anti-CCP2 IgM-positive pts treated with
ABA+MTX. A numerically higher proportion of pts who
seroconverted from anti-CCP2 IgM positive at BL to negative at Mth 12 achieved
Boolean remission versus pts who remained seropositive in the ABA+MTX and ABA
monotherapy arms (Table).
Conclusion: Abatacept in
combination with MTX had greater clinical efficacy in pts
who were anti-CCP2 IgM positive at BL than in those who were anti-CCP2 IgM
negative at BL, and in those who seroconverted over time than those who did
not, suggesting that the impact on ACPA is associated with clinical benefit.
1.
Verpoort
KN, et al. Arthritis Rheum 2006;54:3799–808.
2.
Huizinga
TWJ, et al. Arthritis Rheum 2014;66:S666. Poster 1515.
3.
Emery
P, et al. Ann Rheum Dis 2015:74:19–26.
4.
This
abstract was first presented at the EULAR Congress, 10–13 June 2015, Rome,
Italy (THU0114) and published in the corresponding supplement of Ann Rheum
Dis.
To cite this abstract in AMA style:
Huizinga TWJ, Connolly S, Johnsen A, Zhu J, Furst DE, Bykerk V, Burmester G, Combe B, Wong D, Trouw L, Toes REM, Emery P. Effect of Anti-Cyclic Citrullinated Peptide 2 Immunoglobulin M Serostatus on Efficacy Outcomes Following Treatment with Abatacept Plus Methotrexate [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/effect-of-anti-cyclic-citrullinated-peptide-2-immunoglobulin-m-serostatus-on-efficacy-outcomes-following-treatment-with-abatacept-plus-methotrexate/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-anti-cyclic-citrullinated-peptide-2-immunoglobulin-m-serostatus-on-efficacy-outcomes-following-treatment-with-abatacept-plus-methotrexate/
