Session Information
Date: Sunday, November 10, 2019
Title: 3S076: Patient Outcomes, Preferences, & Attitudes I: Patient Reported Outcomes (833–838)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Treat to target (TTT) is an effective strategy to improve outcomes in rheumatoid arthritis (RA). However, barriers to TTT include frequent clinic visits, poor access to rheumatologists, and poor patient acceptance of treatment escalation. Mobile technology may provide a solution by enabling collection of patient-reported outcomes (PROs) between clinic visits. The objectives of this study were to examine the effects of a mobile application (app) to monitor PROs on: 1) patient satisfaction and 2) disease activity in RA.
Methods: We conducted a 6-month randomized controlled trial of an app + population manager vs. population manager alone in 191 RA patients. Participants in the app group were prompted to answer daily questionnaires on disease activity, function, pain, fatigue, sleep, and mood, using an app. Both groups were assigned a population manager who spoke with participants on the telephone at 6 and 18 weeks. Population managers also communicated with participants in the app group if responses to the daily assessments indicated a sustained increase in disease activity. The analysis followed an intent-to-treat principle. Missing data were imputed using last observation carried forward. To address the aim of improving patient satisfaction, the main outcomes were the global satisfaction score from the Treatment Satisfaction Questionnaire for Medication (TSQM) and the Perceived Efficacy in Patient-Physician Interactions (PEPPI) score. To address the aim of decreasing disease activity, the primary outcome was the Clinical Disease Activity Index (CDAI). Treatment effects were estimated with repeated measures analyses (baseline, 3-month, 6-month), using bootstrapped quantile regression models, adjusted for age, sex, and race.
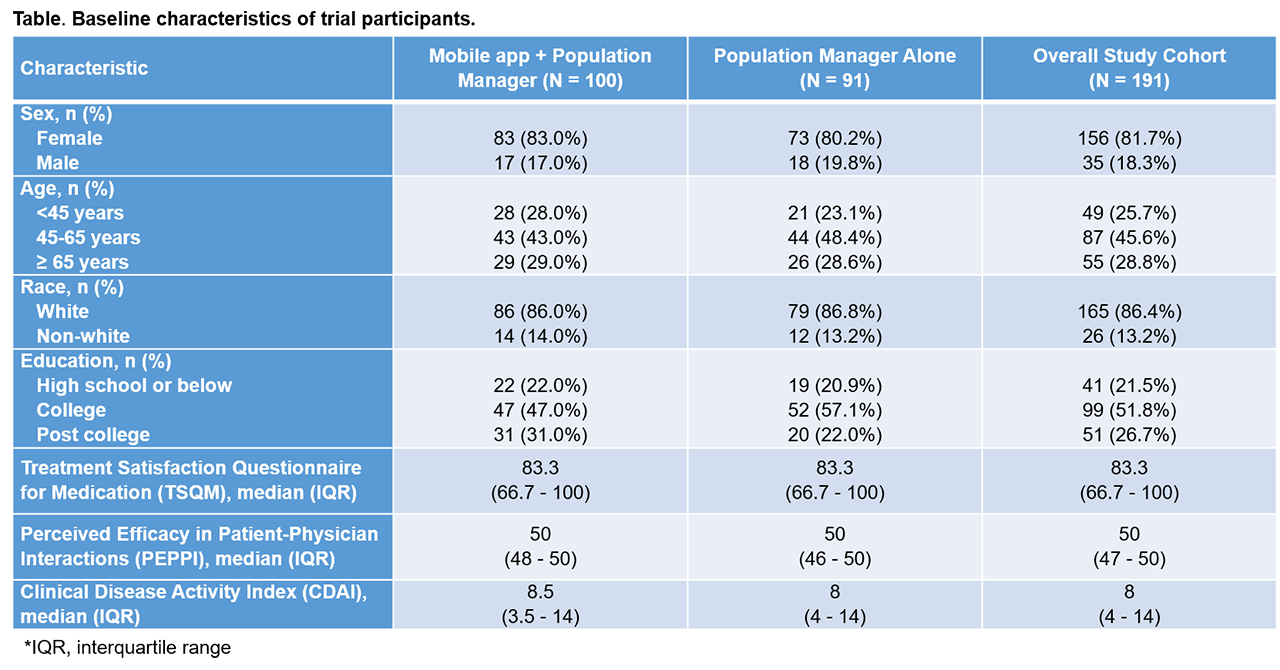
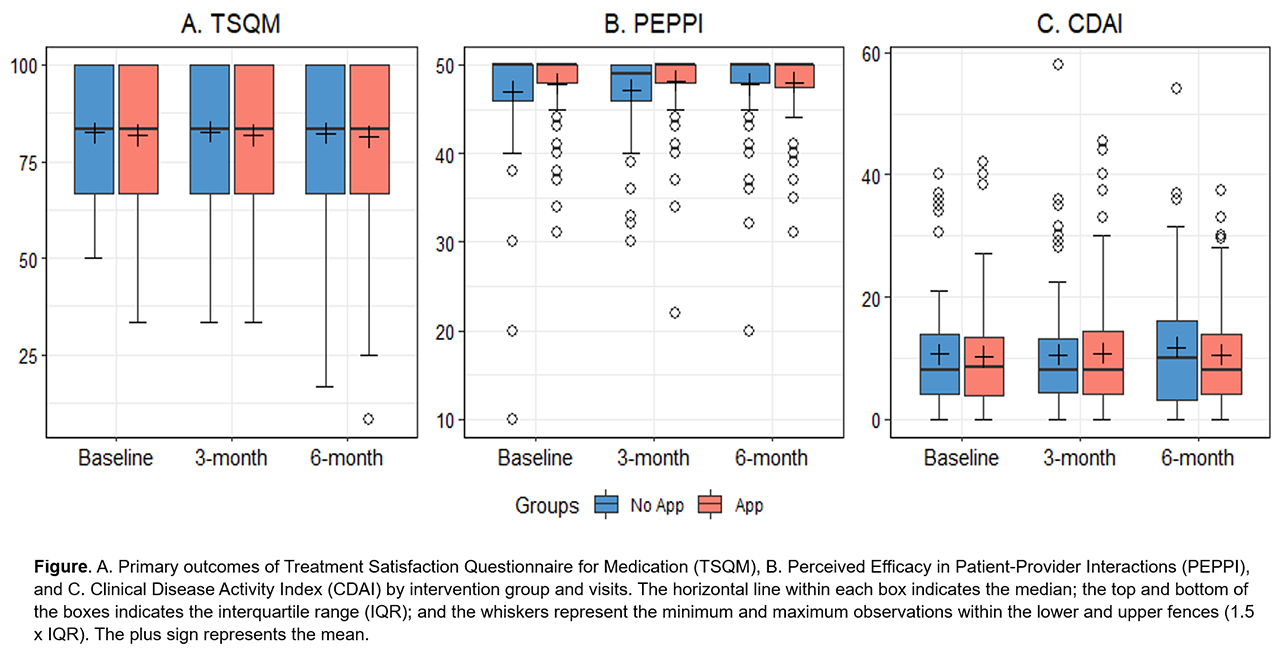
Results: Of the 191 participants, 156 (82%) were women. 29% were at least 65 years old. Baseline global TSQM and PEPPI scores were high, with a median of 83.3 (interquartile range [IQR] 66.7-100) and 50 (IQR 47-50), respectively ( Table ). The median baseline CDAI was 8 (IQR 4-14), indicating low disease activity. After 6 months, median TSQM scores were 83.3 (IQR 66.7-100) in both groups, and median PEPPI scores were 50 (IQR 47-50) in the app group and 50 (IQR 48-50) in the no app group ( Figure ). Median 6-month CDAI scores were 8 (IQR 4-14) in the app group vs. 10 (IQR 3-16) in the no app group. Quantile regression analyses indicated no group (app vs. no app) differences at 6-months in medians of: TSQM β 0.00, 95% confidence interval [CI] -9.18, 9.18; PEPPI β 0.00, 95% CI -0.16, 0.16; and CDAI β -0.50, 95% CI -3.82, 2.82. Adherence with the app was over 75% during the 6-month follow-up.
Conclusion: A mobile app designed to collect PRO data on RA symptoms did not improve patient satisfaction or disease activity. However, baseline satisfaction scores were high and disease activity was low, suggesting ceiling and floor effects. Adherence with the app was strong. Future studies are needed to determine: a) if the app would be beneficial in a population with lower baseline satisfaction and/or more disease activity, and b) if improvements in the app (e.g., voice-enabled system, personalized frequency of data collection) would enhance efficacy.
To cite this abstract in AMA style:
Lee Y, Lu F, Colls J, Murray M, Suh D, Song J, Lee J, Dunlop D, Solomon D. Effect of a Mobile App to Monitor Patient Reported Outcomes in Rheumatoid Arthritis: A Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effect-of-a-mobile-app-to-monitor-patient-reported-outcomes-in-rheumatoid-arthritis-a-randomized-controlled-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-a-mobile-app-to-monitor-patient-reported-outcomes-in-rheumatoid-arthritis-a-randomized-controlled-trial/