Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Diminished IL-2 as well as both quantitative and qualitative abnormalities in regulatory T cells (Treg) are associated with autoimmune diseases including SLE. Efavaleukin alfa is a novel IL-2 mutein Fc fusion protein designed to selectively expand Treg. In healthy subjects, a single dose of efavaleukin alfa was well tolerated and demonstrated Treg selectivity with minimal changes in CD4+ conventional T cells (Tcon), CD8+ T cells, or NK cells (Tchao N Blood 2017). This interim analysis of an ongoing phase 1b study (NCT03451422) reports the safety, tolerability, pharmacokinetics (PK), and pharmacodynamic effects of multiple doses of efavaleukin alfa in patients with SLE.
Methods: Four of five ascending dosing cohorts are reported in this analysis. A total of 29 patients with SLE (age 18-70 years; 82.8% female; SLE diagnosed using SLICC or ACR criteria with ANA ≥1:80 and/or elevated anti-dsDNA antibodies) were randomized to receive efavaleukin alfa or placebo (5:2 ratio for cohorts 1–3; 3:1 ratio for cohort 4) subcutaneously every 2 weeks (Q2W; cohorts 1, 2, and 4) or every week (QW; cohort 3) in addition to standard of care therapy for a total of 12 weeks, with 6 weeks of follow-up. Endpoints assessed in this analysis included treatment-emergent adverse events (TEAEs), serum PK profiles of efavaleukin alfa, changes in numbers of Treg, CD4+ Tcon, CD8+ T cells, and NK cells, and levels of cytokines in peripheral blood.
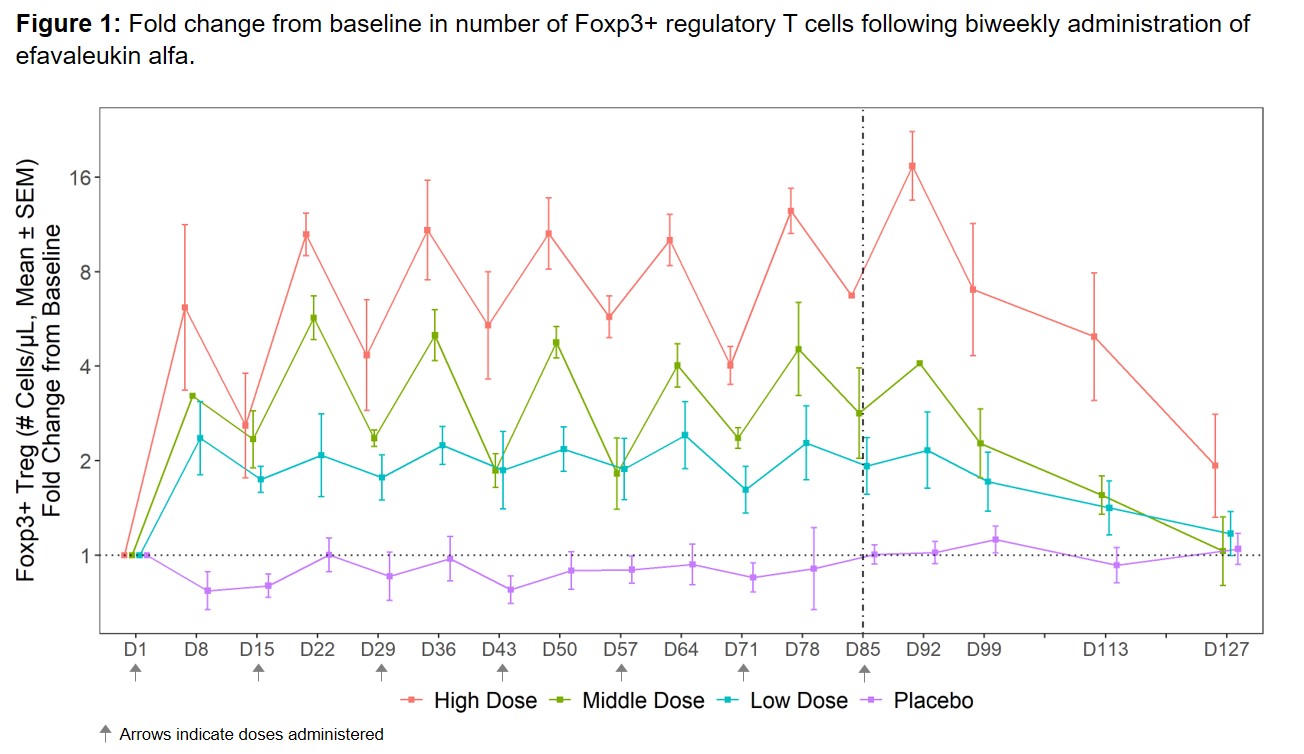
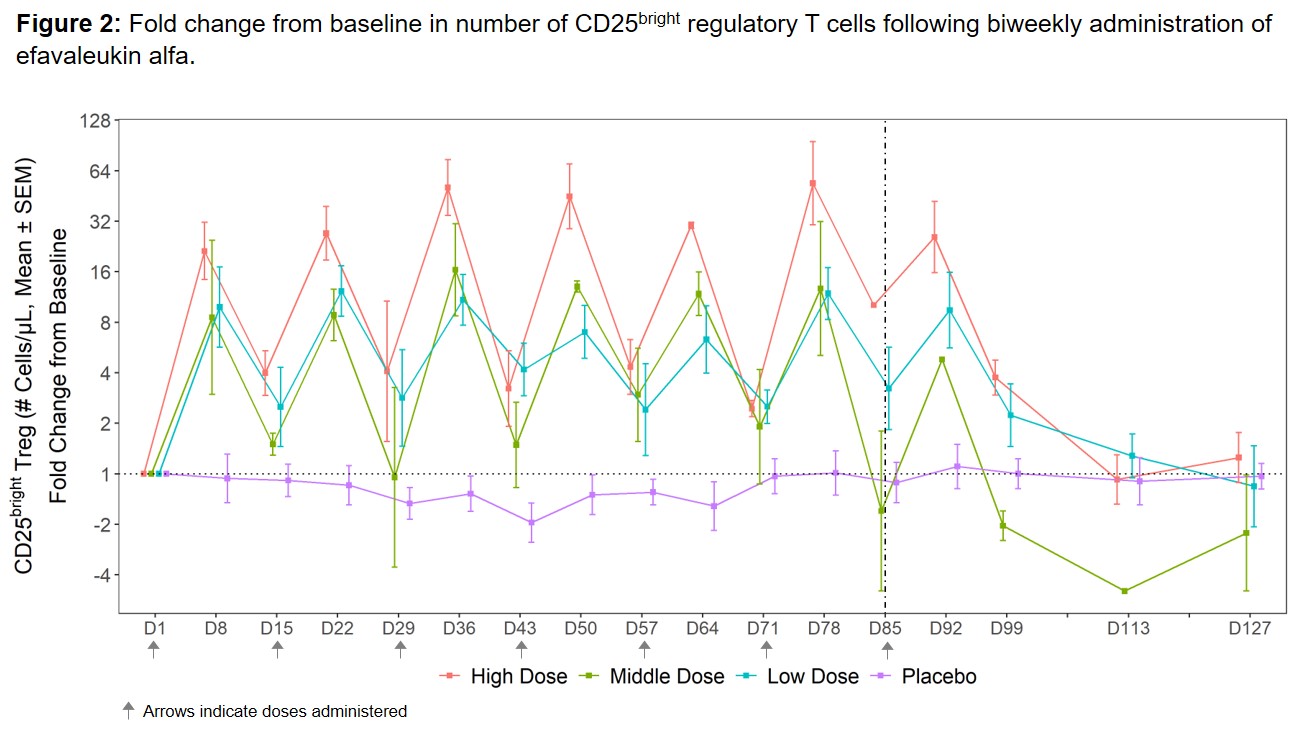
Results: The most commonly reported TEAEs (≥25% of all subjects) included non-serious, mild or moderate (grade 1–2) injection site reactions. No dose-limiting toxicities, treatment-related serious AEs, or deaths were reported. Efavaleukin alfa PK was generally linear and dose-proportional, with a terminal half-life ranging from 18 to 30 hours. Peak Foxp3+ Treg expansion was observed at 8 days post-dose, and the magnitude of the peaks was generally sustained after multiple QW or Q2W doses. Treg numbers remained above baseline for an average of 42 days after the last dose (Figure 1). The mean peak increases in Foxp3+ Treg were 17.4-, 5.7-, 2.4-, and 1.1-fold above baseline for the high, middle, and low Q2W efavaleukin alfa and placebo cohorts, respectively. At the highest dose, the mean peak increase in CD25bright Treg was 53.8-fold above baseline (Figure 2). Treatment with efavaleukin alfa expanded CD31+ recent thymic emigrant and naïve Treg populations; increases in these Treg subsets persisted longer than increases in memory Treg subsets. The majority of expanded Treg expressed markers associated with suppressive activity, including Helios, PD-1, ICOS, CD39, and GITR. There were no meaningful changes in numbers of CD4+ Tcon, CD8+ T cells, or NK cells or serum levels of pro-inflammatory cytokines with treatment.
Conclusion: Multiple doses of efavaleukin alfa were well tolerated in SLE patients. Treatment with efavaleukin alfa resulted in robust and prolonged dose-dependent Treg expansion, with minimal changes in other IL-2-responsive cells highlighting pharmacodynamic selectivity. These findings confirm and extend previous results in healthy subjects and support the ongoing phase 2b adaptive randomized controlled trial in patients with SLE.
To cite this abstract in AMA style:
Tchao N, Amouzadeh H, Sarkar N, Chow V, Hu X, Kroenke M, Wang H, Zhang R, Gorski K, Furie R, Kivitz A, Cohen S. Efavaleukin Alfa, a Novel IL-2 Mutein, Selectively Expands Regulatory T Cells in Patients with SLE: Interim Results of a Phase 1b Multiple Ascending Dose Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efavaleukin-alfa-a-novel-il-2-mutein-selectively-expands-regulatory-t-cells-in-patients-with-sle-interim-results-of-a-phase-1b-multiple-ascending-dose-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efavaleukin-alfa-a-novel-il-2-mutein-selectively-expands-regulatory-t-cells-in-patients-with-sle-interim-results-of-a-phase-1b-multiple-ascending-dose-study/