Session Information
Date: Sunday, November 8, 2015
Title: Health Services Research Poster I: Diagnosis, Management and Treatment Strategies
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
RA patients
with inadequate treatment response often cycle non-biologic DMARDs before
initiating a biologic DMARD. Early and aggressive treatment is a key feature of
a treat-to-target approach and a previous study showed that switching to a
biologic DMARD compared to cycling another non-biologic DMARD resulted in
improved clinical outcomes (Dewitt et al., 2013). This study assessed the
economic outcomes and treatment patterns among patients who used 1, 2, or ≥3
non-biologic DMARD(s) before receiving a biologic therapy.
Methods:
Adult
patients with ≥2 RA diagnoses (International Classification of Diseases: 714.xx),
≥1 claim for a non-biologic DMARD, and ≥1 claim for a biologic DMARD
were identified from a large commercial claims database (2008-2013). The initiation
date of the first biologic DMARD was defined as the index date. Based on the
number of distinct non-biologic DMARDs initiated between the first RA diagnosis
and the index date, patients were classified into three cohorts: those who used
1, 2, or ≥3 non-biologic DMARDs. Baseline characteristics were measured 6
months pre-index date and compared between the three cohorts. All-cause healthcare
costs (in 2014 USD) were compared in the follow-up period (12 months post
biologic initiation) using multivariable gamma models adjusting for baseline
characteristics. Discontinuation of the index biologic DMARD and time to
switching to a new DMARD were compared using multivariable Cox proportional
hazards models.
Results:
The 1,
2, and ≥3 non-biologic DMARD cohorts included 6,215, 3,227, and 976
patients, respectively. At baseline, patients in the ≥3 non-biologic
cohort had the least severe RA, as indicated by the lowest claims-based index
for RA severity (CIRAS) score (1 vs 2 vs ≥3: 6.1 vs 5.9 vs 5.8). During
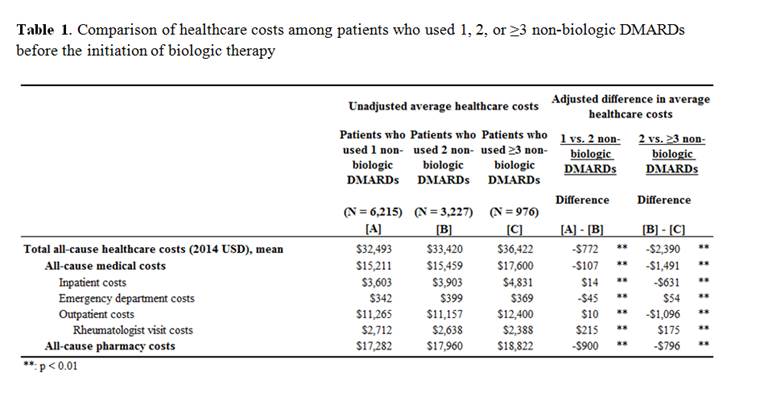
the study period, there was a significant association between number of
non-biologic DMARDs and higher all-cause total healthcare costs (adjusted mean
difference: 1 vs 2: $772, p<0.001; 2 vs ≥3: $2,390, p<0.001); the
all-cause medical and pharmacy costs were also significantly higher with the increasing
number of non-biologics (Table 1). Patients who cycled more non-biologic DMARDs
were also more likely to switch treatment after biologic initiation (1 vs 2:
adjusted hazard ratio (HR)=0.89, p=0.005; 2 vs ≥3: adjusted HR=0.89,
p=0.087). There were no differences in index biologic discontinuation between
the 3 cohorts.
Conclusion:
RA
patients who cycled more non-biologic DMARDs had increased economic burden in
the 12 months following biologic initiation and were more likely to switch
therapy. These
results highlight
the importance of timely switching to biologic DMARDs for the treatment of RA.
To cite this abstract in AMA style:
Li N, Betts K, Griffith J, Ristovska L, Douglas K, Ganguli A. Economic Burden and Treatment Patterns of Cycling Between Non-Biologic Disease Modifying Anti-Rheumatic Drugs (DMARDs) Among Patients with Rheumatoid Arthritis (RA) [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/economic-burden-and-treatment-patterns-of-cycling-between-non-biologic-disease-modifying-anti-rheumatic-drugs-dmards-among-patients-with-rheumatoid-arthritis-ra/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/economic-burden-and-treatment-patterns-of-cycling-between-non-biologic-disease-modifying-anti-rheumatic-drugs-dmards-among-patients-with-rheumatoid-arthritis-ra/