Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
We used data from a large randomized controlled clinical trial of an effective biologic (golimumab, GO-BEFORE study) to compare the associations of disease activity and disease severity with two imaging techniques to measure joint erosion: 1) early progression in rheumatoid arthritis magnetic resonance imaging scores (RAMRIS) and 2) radiographic progression. We subsequently assessed the potential impact of incorporating these MRI scores in clinical trial design.
Methods
MRI of the dominant hand was performed and RAMRIS scores were determined at baseline, week 12 and week 24. van der Heijde-Sharp (vdHS) scores were determined for x-rays at baseline and week 52. Progression in vdHS and RAMRIS erosion scores were defined as a change of >0.5. Associations between X-ray and MRI outcomes with clinical features associated with severe disease and structural damage were evaluated to assess convergent validity. Iterative Wilcoxon ranksum tests assessed the sample size requirements to detect a significant difference in the change in structural damage score between combination therapy (methotrexate+golimumab) and methotrexate monotherapy. Sample size calculations were also performed based on dichotomous progression outcomes.
Results
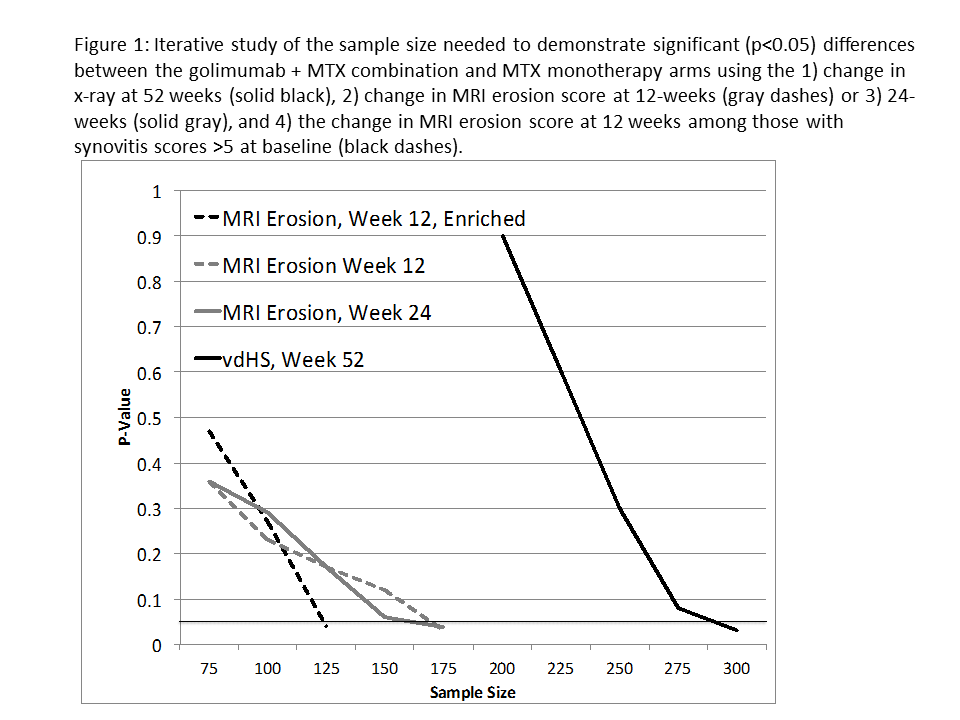
MRI progression at 12 and 24 weeks was associated with greater DAS28(CRP), CRP, and vdHS at baseline, and greater HAQ scores at 2-years (Table 1). These associations were similar in magnitude to those seen with X-ray progression at 1-year. Ranksum testing for differences in the change in structural damage between treatment and controls arms achieved significance (p<0.05) with fewer total study subjects when MRI erosion score was the outcome assessed (175 for MRI at 12/24 weeks v. 300 subjects for ΔvdHS at 52 weeks) (Figure 1). When the study sample was enriched with subjects with a synovitis score >5 (a level previously associated with MRI progression) at baseline, the study sample further decreased to 125. Sample size calculations demonstrated that a study enriched with 117 subjects with synovitis scores >5 would have 80% power to detect a difference in the proportion of subjects progressing on MRI at 12 weeks. A sample size calculation based on an unenriched population and using 1-year x-ray progression as the outcome estimated a study size of 470 subjects.
Conclusion
Early MRI erosion score outcomes have convergent validity comparable to that of 52-week x-ray progression. Use of MRI in clinical trials would decrease sample sizes and reduce length of follow-up for studies assessing differences in structural damage progression between groups.
|
Table 1: Clinical characteristics of MRI/x-ray progressors and non-progressors within the MRI sub-study (convergent validity).
|
||||||
|
|
MRI 24 Weeks (N=238)
|
|
X-ray 52 Weeks (N=238)
|
|||
|
|
Progression (N=52) |
No Progression (N=186) |
P Value |
Progression (N=62) |
No Progression (N=176) |
P Value |
|
Baseline DAS28(CRP)
|
5.81 (1.13) |
5.53 (1.06) |
0.1 |
5.79 (1.10) |
5.52 (1.06) |
0.09 |
|
Baseline CRP
|
1.8 (0.95, 3.4) |
1 (0.4, 2.5) |
0.002 |
2 (0.8, 4.2) |
1 (0.4, 2.4) |
0.001 |
|
Baseline vdHS
|
15 (3.25, 29.8) |
4.5 (2, 13) |
0.002 |
9 (2.9, 26) |
4.5 (2, 15) |
0.01 |
|
ΔHAQ Score (2-years)*
|
0.036 (0.78) |
-0.14 (0.59) |
0.08 |
-0.054 (0.74) |
-0.12 (0.61) |
0.5 |
|
24 Week ACR50, N (%)
|
14 (28%) |
86 (47%) |
0.01 |
21 (34%) |
79 (45%) |
0.1 |
* Adjusted for baseline HAQ
Disclosure:
J. Baker,
None;
P. G. Conaghan,
Abbvie,
8,
Merck Human Health,
8,
Novartis Pharmaceutical Corporation,
8,
Pfizer Inc,
8,
Roche Pharmaceuticals,
8,
UCB,
8;
P. Emery,
AbbVie, Bristol-Myers Squibb (BMS), MSD, Novartis, Pfizer Inc, Roche, and UCB Pharma,
2,
AbbVie, Bristol-Myers Squibb (BMS), MSD, Novartis, Pfizer Inc, Roche, and UCB Pharma,
5;
D. Baker,
Janssen Research & Development, LLC.,
3;
M. Østergaard,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-mri-endpoints-provide-a-valid-measure-of-structural-damage-while-reducing-study-duration-and-participant-numbers-in-rheumatoid-arthritis-clinical-trials/