Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
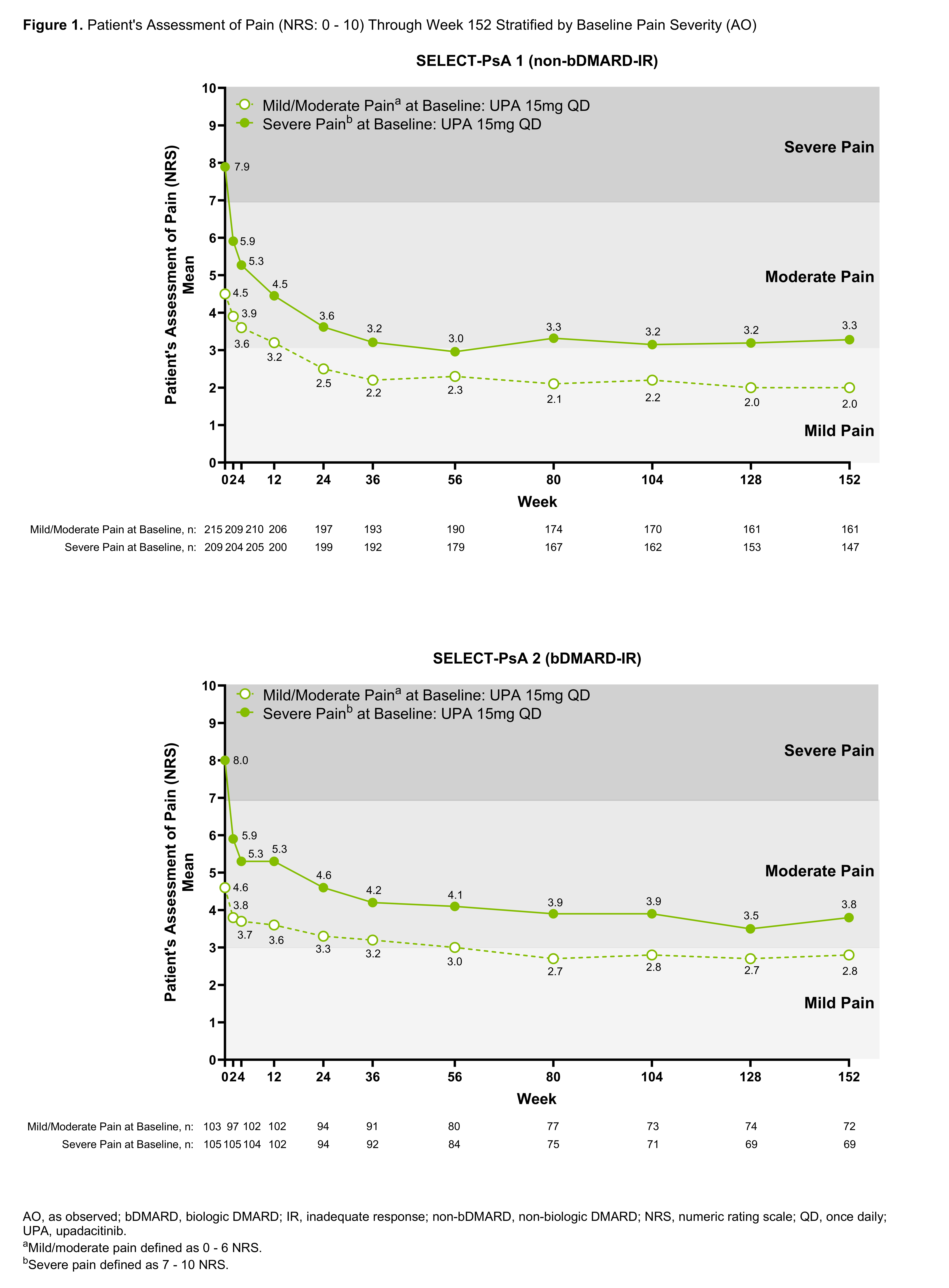
Background/Purpose: Severe musculoskeletal manifestations of PsA, particularly joint pain, can result in reduced physical function, decreased quality of life, and progressive and irreversible joint damage.1,2 Upadacitinib (15mg; UPA15), an oral JAK inhibitor, has shown to rapidly and meaningfully reduce pain in patients with active PsA and inadequate response (IR) to non-biologic DMARDs (non-bDMARD-IR, including csDMARDs or apremilast) or biologic DMARDs (bDMARD-IR).3 However, there is limited evidence of how rapid pain improvement impacts long-term disease control and quality of life. This analysis reports the improvement in pain by baseline pain severity and the impact of early pain improvement on long-term, stringent disease control targets and patient-reported outcomes in patients with PsA treated with UPA15 through 152 wks.
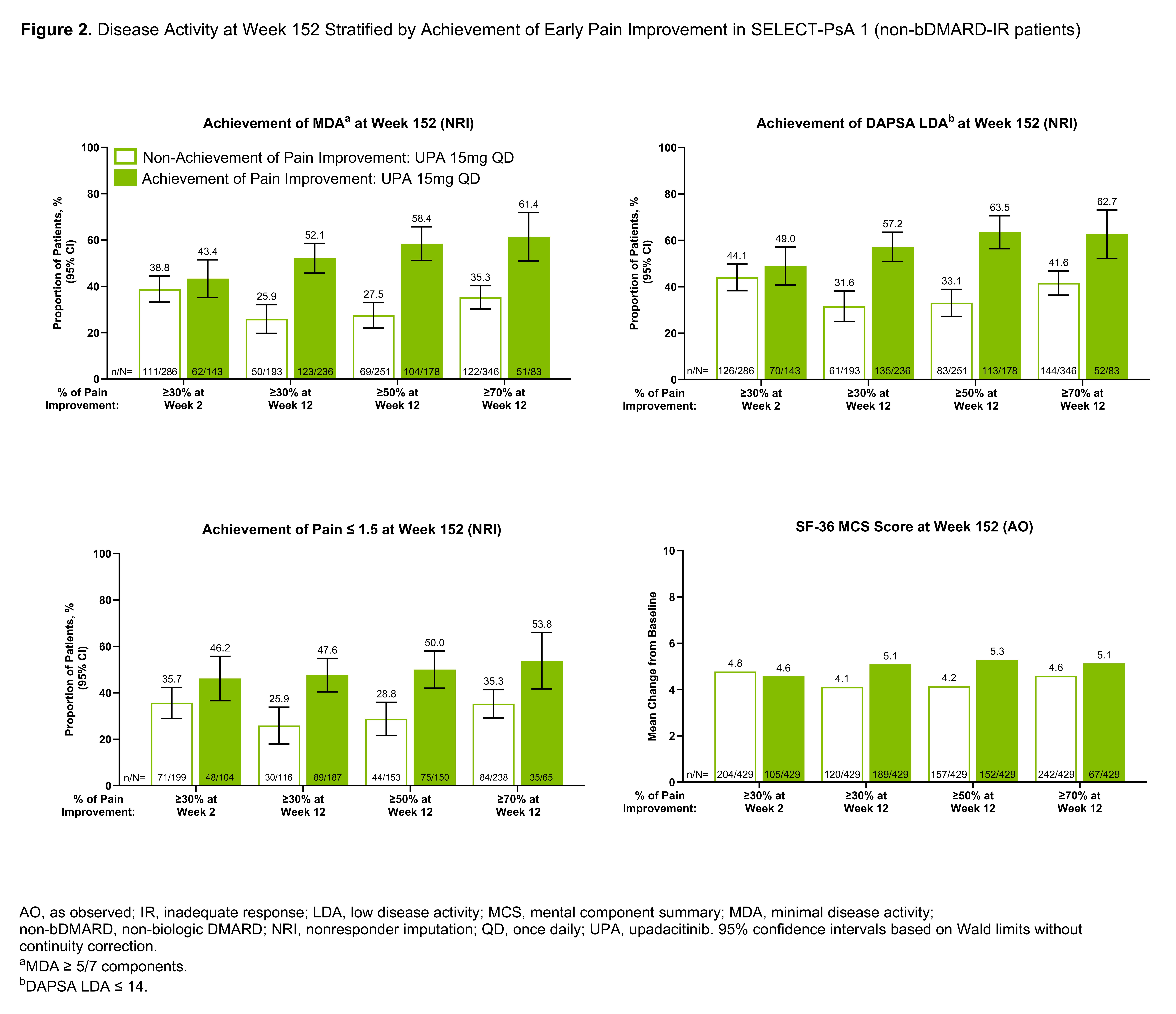
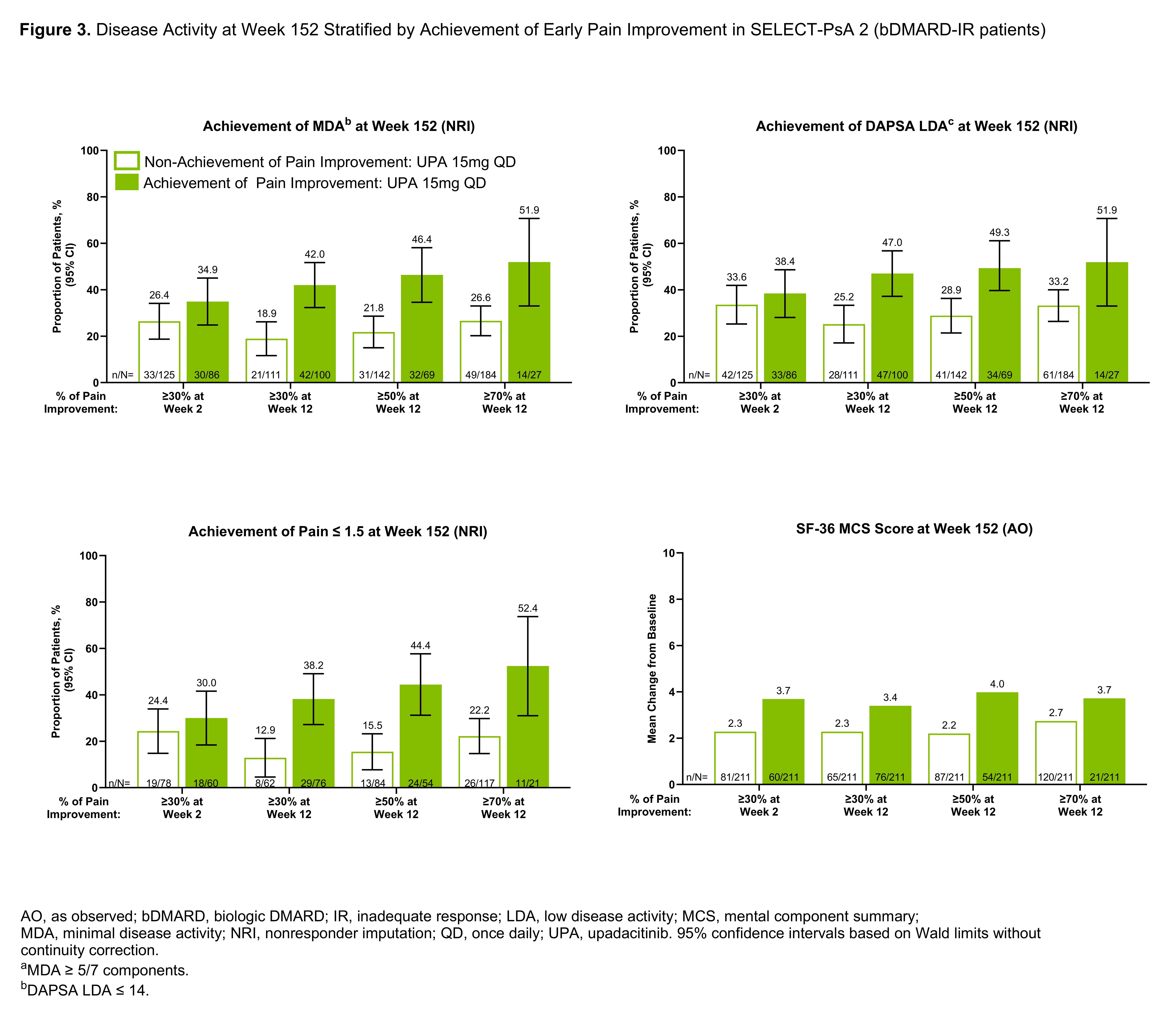
Methods: In this post hoc analysis, patients randomized to UPA15 in the phase 3 trials SELECT-PsA 1 (N=429; non-bDMARD-IR patients) and SELECT-PsA 2 (N=211; bDMARD-IR patients) were analyzed separately.4,5 Mean pain scores (as observed, AO) over 152 wks were calculated in patients with mild/moderate (0-3/4-6) and severe (7-10) baseline pain severity (numeric rating scale, NRS: 0-10). Patients were also stratified by achievement/nonachievement of early pain improvement (≥30% at wk 2 and ≥30/50/70% at wk 12); for each group, achievement of MDA, DAPSA low disease activity (LDA; ≤14), or pain ≤1.5 (NRS) (all reported as nonresponder imputation) and mean change from baseline in SF-36 mental component summary (SF-36 MCS; reported AO) was calculated at wk 152.
Results: For patients with severe pain at baseline, mean pain scores rapidly decreased to moderate levels in the first 2 wks of treatment with UPA15, with additional reductions and achievement of mild/moderate levels by wk 152 in both non-bDMARD-IR (wk 152 mean: 3.3) and bDMARD-IR patients (3.8) (Figure 1). For patients with mild/moderate pain at baseline, mean pain scores decreased and were maintained to mild levels through wk 152 in non-bDMARD-IR (2.0) and bDMARD-IR (2.8) patients. In both studies, a larger proportion of patients with early pain improvements achieved MDA, DAPSA LDA, and pain ≤1.5 at wk 152, compared to patients without early pain improvements (Figure 2 and Figure 3). Additionally, bDMARD-IR patients with early pain improvements reported greater improvements in SF-36 MCS score at wk 152 compared to those without early improvements, with smaller differences observed in non-bDMARD-IR patients.
Conclusion: In non-bDMARD-IR and bDMARD-IR PsA patients, reductions in pain score occurred rapidly and were maintained through 152 wks of treatment with UPA15, regardless of pain severity at baseline. More patients with early pain improvements achieved long-term, stringent disease control targets and quality of life outcomes compared to patients without early pain improvements, suggesting that rapid pain improvement may be a positive indicator of long-term disease control.
1. Coates LC, et al. Health Qual Life Outcomes. 2020;18:173.
2. Ogdie A, et al. Rheum & Therapy. 2021;9:735-51.
3. McInnes IB, et al. RMD Open. 2022;8:e002049.
4. McInnes, IB et al. N Engl J Med. 2021;384:1227-39.
5. Mease PJ, et al. Ann Rheum Dis. 2021;80:312-20.
Disclosures: P. Taylor: AbbVie, 2, Acelyrin Inc., 2, Biogen, 2, Eli Lilly, 2, Fresenius, 2, Galapagos, 2, 12, Participation on a Data Safety Monitoring Board/Advisory Board, Gilead, 2, GSK, 2, Immunovant, 12, Participation on a Data Safety Monitoring Board/Advisory Board, Janssen, 2, Kymab, 12, Participation on a Data Safety Monitoring Board/Advisory Board, Nordic Pharma, 2, Pfizer, 2, Sanofi, 12, Participation on a Data Safety Monitoring Board/Advisory Board, UCB Pharma, 2; L. Eder: AbbVie, 2, 5, 6, Bristol-Myers Squibb (BMS), 2, Eli Lilly, 2, 5, Fresenius Kabi, 5, Johnson & Johnson, 2, 5, Novartis, 1, 5, Pfizer, 5, 6, UCB, 5, 6; Y. Klionsky: AbbVie/Abbott, 5, Amgen, 2, AstraZeneca, 5, 6, Eli Lilly, 2, Janssen, 6, MediQ, 2; F. Proft: AbbVie, 2, 6, Amgen, 2, 6, BMS, 2, 6, Celgene, 2, 6, Eli Lilly, 5, Galapagos, 2, 6, Hexal, 2, 6, Janssen, 2, 6, Medscape, 2, 5, MSD, 2, 6, Novartis, 2, 5, 6, Pfizer, 2, 6, Roche, 2, 6, UCB Pharma, 2, 5, 6; T. Iyile: AbbVie, 3, 11; E. Mancl: AbbVie, 3, 11; P. Magalhaes Reis Nakasato: AbbVie, 3, 11; X. Ye: AbbVie, 3, 11; L. Zhou: AbbVie, 3, 11; P. Mease: AbbVie, 2, 5, Aclaris Therapeutics, 2, 5, Aclyrin, 2, 5, Amgen, 2, 5, Boehringer Ingelheim, 2, 5, Bristol Myers Squibb, 2, 5, CorEvitas, 2, 5, Galápagos, 2, 5, Gilead, 2, 5, Inmagene, 2, 5, Janssen, 2, 5, Lilly, 2, 5, MoonLake Immunotherapeutics, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, Sun Pharma, 2, 5, UCB, 2, 5.
To cite this abstract in AMA style:
Taylor P, Eder L, Klionsky Y, Proft F, Iyile T, Mancl E, Magalhaes Reis Nakasato P, Ye X, Zhou L, Mease P. Early Improvement of Pain, Long-Term Disease Control, and Quality of Life Outcomes in Patients with Psoriatic Arthritis Treated with Upadacitinib [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/early-improvement-of-pain-long-term-disease-control-and-quality-of-life-outcomes-in-patients-with-psoriatic-arthritis-treated-with-upadacitinib/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-improvement-of-pain-long-term-disease-control-and-quality-of-life-outcomes-in-patients-with-psoriatic-arthritis-treated-with-upadacitinib/