Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: In the absence of reliable serological and/or imaging biomarkers for early psoriatic arthritis (PsA) and an existing diagnostic delay there is a need for screening tools for detection of early PsA. While different validated screening/referral tools focusing on peripheral manifestations of PsA exist1, validated referral algorithms for PsA with axial involvement (axPsA) are missing. In this prospective, multicenter study we applied a dermatologist-centered screening tool to identify signs of axial involvement among patients with psoriasis (PsO) attending dermatology clinics.
Methods: Consecutive patients with PsO were screened by their dermatologist for eligibility for referral to a specialized rheumatology clinic. Eligible patients were ≥ 18 years with a confirmed diagnosis of PsO who reported having chronic back pain (≥ 3 months) with onset prior to 45 years of age and who had not been treated with any biologic or targeted synthetic DMARD in the 12 weeks prior to screening. For those patients who qualified for referral and attended the rheumatology clinic, a rheumatologic investigation including clinical, laboratory and genetic assessments as well as imaging with conventional radiography and MRI of sacroiliac joints and spine was performed. The primary outcome of the study was the proportion of patients diagnosed with axPsA among all referred PsO patients.
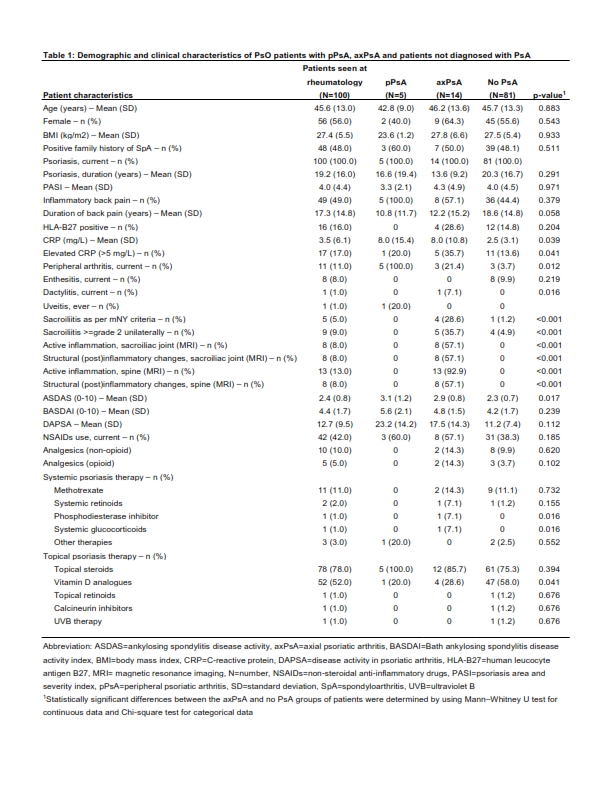
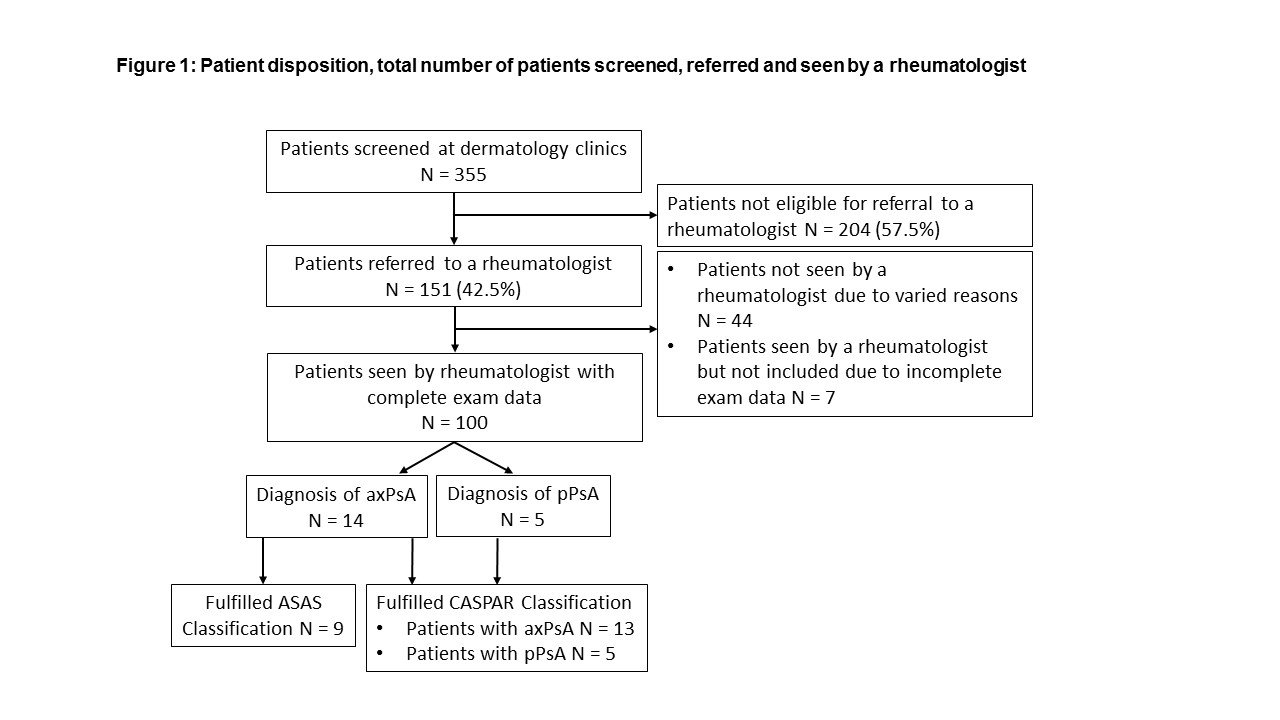
Results: In total 355 patients were screened at 14 dermatology sites, of whom 151 (42.5%) qualified for referral to rheumatology clinic. Rheumatologists ultimately examined 100 (28.2%) patients (Figure 1). The diagnosis of axPsA was confirmed in 14 patients (14%) (3/14 with both, axial and peripheral involvement) and the diagnosis of peripheral PsA (pPsA) without axial involvement was made in five patients (5%). 81 (81%) patients were diagnosed with neither pPsA nor axPsA. Clinical, lab and imaging characteristics of the study population, as well as subgroups with and without axial involvement, are presented in Table 1. All patients diagnosed with axPsA had active inflammatory and/or structural (post) inflammatory changes in the sacroiliac joints and/or spine on imaging. In five (35.7%) patients, MRI changes indicative of axial involvement were found only in the spine. The Assessment of SpondyloArthritis international Society (ASAS) classification criteria for axSpA were fulfilled in nine (64.3%) of the patients diagnosed with axPsA. All but one patient diagnosed with PsA (13/14 with axPsA and 5/5 with pPsA) fulfilled the Classification Criteria for Psoriatic Arthritis (CASPAR) for PsA as illustrated in Figure 1.
Conclusion: Our study revealed that applying a dermatologist-centered screening tool is useful for the detection of axPsA and pPsA in PsO patients at-risk. These results provide valuable real-world insights into the possibility of diagnosing axPsA and pPsA early with the ultimate goal of improving the care and quality of life of patients living with the disease.
Mishra S et al. BR J Dermatol. 2017;176(3):765-770.
Acknowledgements: C. Höppner, R. Bolce, D. Sandoval, H. Russ, B. Muche, J. Rademacher, H. Haibel, L. Spiller and all cooperating dermatologists.
To cite this abstract in AMA style:
Proft F, Lüders S, Hunter T, Luna G, Rios Rodriguez V, Protopopov M, Meier K, Kokolakis G, Ghoreschi K, Poddubnyy D. Early Identification of Psoriatic Arthritis with Axial Involvement Among Patients with Psoriasis: A Prospective Multicenter Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/early-identification-of-psoriatic-arthritis-with-axial-involvement-among-patients-with-psoriasis-a-prospective-multicenter-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-identification-of-psoriatic-arthritis-with-axial-involvement-among-patients-with-psoriasis-a-prospective-multicenter-study/