Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Fatigue is commonly reported by PsA patients (pts) and contributes to disease burden. The fully human IL-23p19-subunit inhibitor guselkumab (GUS) induces clinically meaningful and sustained fatigue improvements through 1y, with GUS exhibiting a substantial direct effect on fatigue, independent of its impact on other clinical outcomes1,2. We previously showed that early fatigue response predicted clinically meaningful improvement (CMI) in fatigue at 2y3. In a sub-population of PsA pts with substantial fatigue at baseline (BL), relationships between early fatigue improvement and disease control at 1y were assessed.
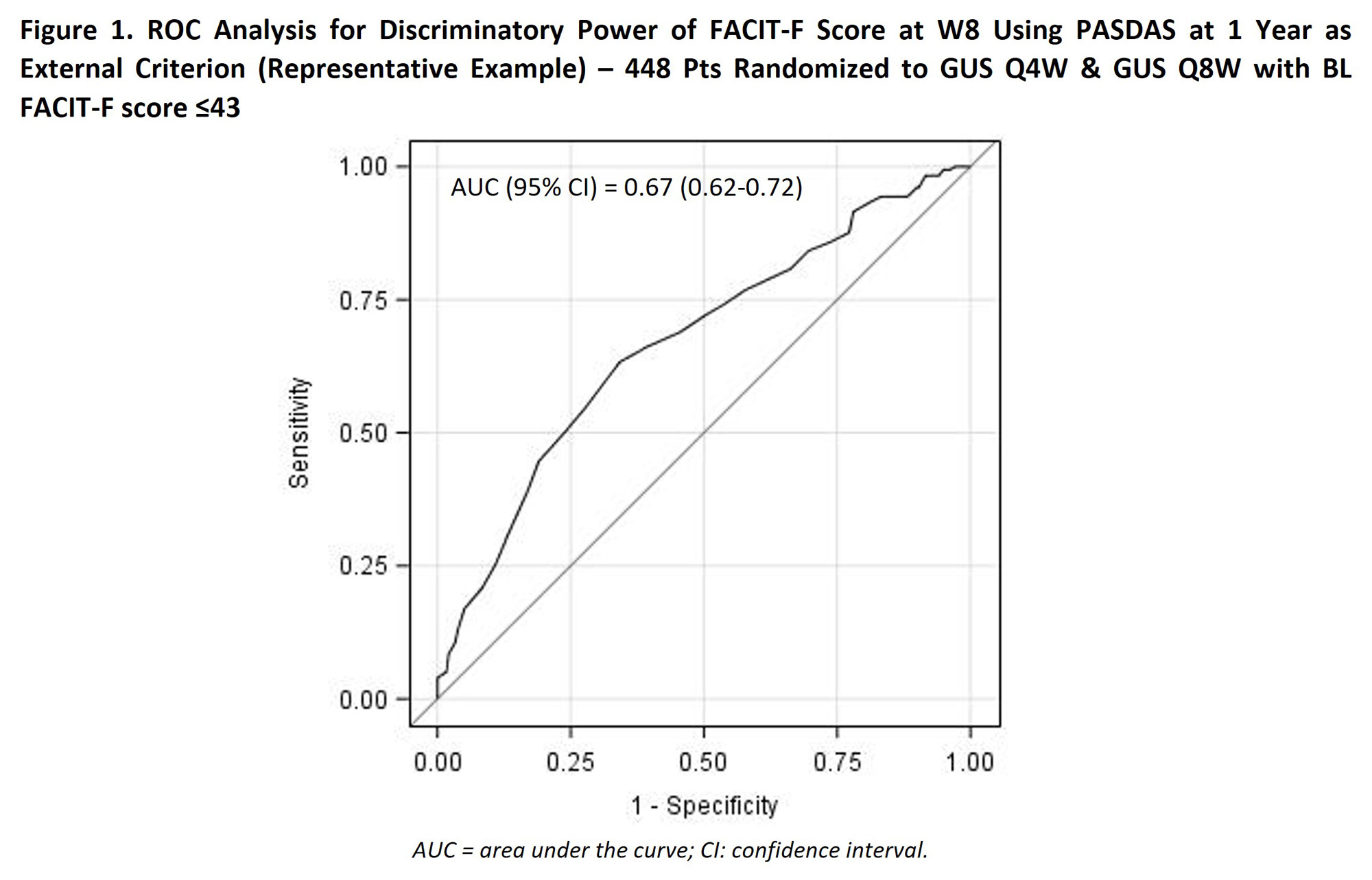
Methods: DISCOVER-2 enrolled bionaïve adults with active PsA (≥5 swollen & ≥5 tender joints, CRP ≥0.6 mg/dl). Pts were randomized (1:1:1) to GUS 100mg every 4 weeks (Q4W); GUS 100mg at W0, W4, Q8W; or placebo (PBO). Pts with BL fatigue below normative levels (Functional Assessment of Chronic Illness Therapy – Fatigue [FACIT-F] score ≤43)3 were included in the analysis. Receiver operating characteristic (ROC) analyses pooling GUS Q4W and Q8W utilized Youden’s index to determine optimal FACIT-F cutoffs at W8 (1st post-BL timepoint assessed) for predicting 1y achievement of ACR50/70, Disease Activity (DA) Index for PsA (DAPSA) ≤14 (in pts with BL DAPSA >14), clinical (c) DAPSA ≤13 (in pts with BL cDAPSA >13), PsA DA Score (PASDAS) ≤3.2 (in pts with BL PASDAS >3.2), HAQ-Disability Index (HAQ-DI) response (improvement ≥0.35 in pts with BL HAQ-DI ≥0.35), HAQ-DI ≤0.5 (in pts with BL HAQ-DI >0.5), and MDA. The association of identified and established FACIT-F cutoffs (≥4-point improvement & score >43) with 1y response was assessed via BL-adjusted logistic regression.
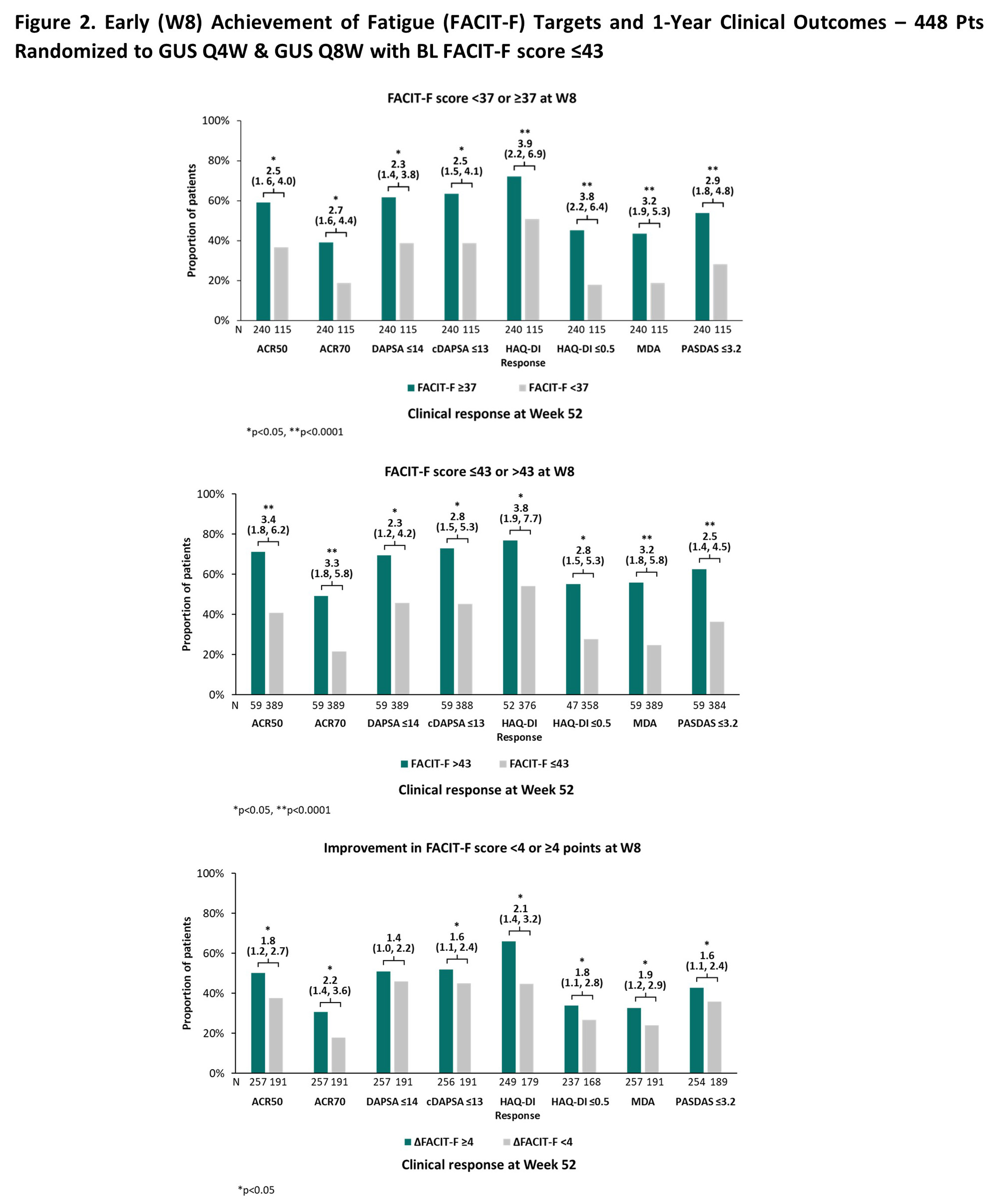
Results: In this sub-population of 448/493 GUS-randomized bionaïve PsA pts with BL FACIT-F score ≤43, the mean (SD) BL FACIT-F level of 28.4 (8.7) indicated severe fatigue. ROC analysis (Figure 1) identified optimal FACIT-F cutoffs at W8 for predicting 1y disease control with GUS ranging from ≥36 (ACR50, DAPSA≤14, cDAPSA≤13, HAQ-DI≤0.5, PASDAS≤3.2) to ≥41 (HAQ-DI response); intermediary cutoffs were identified for remaining endpoints. The FACIT-F ≥37 cutoff was selected for further analyses as the lowest score (highest fatigue) with a significantly higher probability of achievement with GUS vs PBO (32% vs 24%; p=0.0445).
In pts with BL FACIT-F < 37, W8 FACIT-F ≥37 achievement with GUS associated with significantly higher odds of clinical response at 1y (nominal p< 0.05; odds ratio range: 2.3-3.9) (Figure 2). W8 achievement of normative FACIT-F levels or FACIT-F CMI also associated with significantly greater clinical response rates at 1y.
Conclusion: In this sub-population of bionaïve pts with active PsA enriched for high fatigue levels, early achievement of FACIT-F endpoints with GUS associated with higher rates of clinical response and improved/normalized physical function at 1y. Results of this post hoc analysis further underscore the importance of early improvement in patient-reported outcomes such as fatigue on the trajectory of long-term pt outcome.
References:
1. Ritchlin CT. RMD Open. 2022;8;e002195
2. Rahman P. Arthritis Res Ther. 2021;23:190
3. Gladman D. Arthritis Rheumatol. 2022;74 (suppl 9)
To cite this abstract in AMA style:
Gladman D, Baraliakos X, Starr M, Ranza R, Rampakakis E, shiff N, Nantel F, Han C, Ostor A, Mease P. Early Fatigue Improvement with Guselkumab Associates with Longer Term Disease Control in Patients with Active Psoriatic Arthritis Reporting Substantial Fatigue: Post Hoc Analyses of a Sub-Population of a Phase 3, Randomized, Controlled Trial of Guselkumab in Biologic-Naïve Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/early-fatigue-improvement-with-guselkumab-associates-with-longer-term-disease-control-in-patients-with-active-psoriatic-arthritis-reporting-substantial-fatigue-post-hoc-analyses-of-a-sub-population-o/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-fatigue-improvement-with-guselkumab-associates-with-longer-term-disease-control-in-patients-with-active-psoriatic-arthritis-reporting-substantial-fatigue-post-hoc-analyses-of-a-sub-population-o/