Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Lupus nephritis (LN) occurs in over 50% of the systemic lupus erythematosus (SLE) patients. It remains an independent risk factor for mortality, with deaths from LN increasing from 2015-2020. Progression to chronic kidney disease occurs from both lupus specific and non-specific factors, that are due to general mechanisms that occur in many types of chronic kidney disease. In patients with type 2 diabetes and albuminuric kidney disease the risk of kidney failure and cardiovascular events was lower with SGLT2 inhibitors (SGLT2i) than placebo after 2.6 years median follow up. We examined changes in trajectories of eGFR and proteinuria after starting SGLT2i among patients with SLE.
Methods: 45 SLE patients who started taking SGLT2i were included:87% female; 35.5% Caucasian, 55.5% African-American, 9% East Asian; mean age when started on SGLT2i was 47.2 ± 13.4 yrs (mean ± SD). SGLT2i was started for standard of care (diabetes mellitus, chronic kidney disease, or both). eGFR was calculated using the 2021 CKD-EPI creatinine equation. eGFR and Urine protein/creatine ratio were measured at every quarterly visit.The analysis was based on clinical visits up to three years prior to starting SGLT2i and all clinical visits after starting SGLT2i. The mean follow-up time prior to starting SGLT2i was 1.9 years with 5 patients contributing no pre-SGLT2i follow-up. The mean follow-up time after starting SGLT2i was 1.1 years with 2 patients contributing no post SGLT2i follow-up. For each outcome (GFR and urine protein), we estimated each person’s trajectory before and after starting SGLT2i using a mixed effects longitudinal regression model with random person-specific slopes and intercepts.
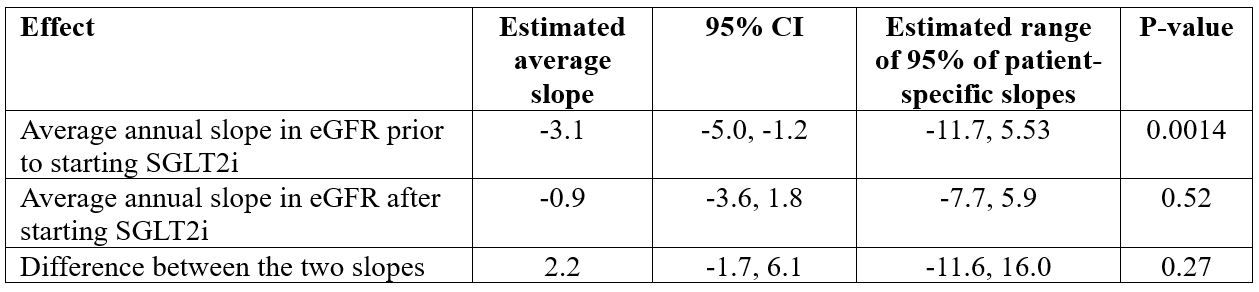
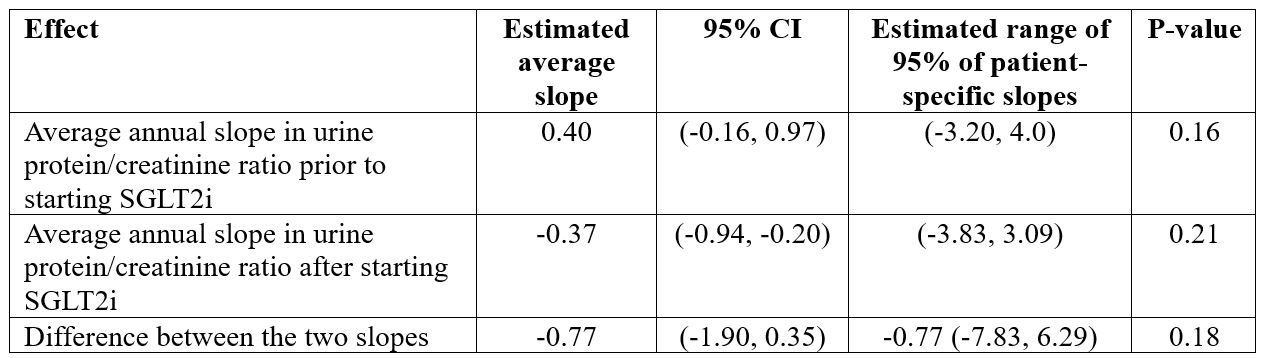
Results: Prior to starting SGLT2i, the average annual change in eGFR was a decline of 3.1 (P=0.0014, Table 1). After starting SGLT2i, the average annual change in eGFR was estimated to be a decline of 0.9. The difference between these two degrees of decline did not reach statistical significance (p=0.27). Prior to starting SGLT2i, the average annual change in urine protein-creatinine ratio was estimated to be an increase of 0.40 (p=0.16, Table 2). However, after starting SGLT2i, the average annual change was estimated to be a decline of 0.37. Again, the difference between these two slopes was not statistically significant (p=0.18).
Conclusion: SLE patients were excluded from the canagliflozin CREDENCE trial (Perkovic V et al. NEJM 380:2295, 2019). Thus, there is a crucial need for data on SGLT2i use in SLE. Like the CREDENCE trial, we observed a reduction in decline in eGFR after starting SGLT2i, however, this reduction was not statistically significant. Similarly, like the CREDENCE trial, we observed an improvement in urine protein/creatinine ratio after starting SGLT2i, but again, our observed difference did not achieve statistical significance. In the CREDENCE trial, reduction in proteinuria was observed by 6 months, and then plateaued. Thus, early experience suggested marginal benefit of SGLT2i in SLE. Additional patients and longer follow-up are needed for a more definite conclusion regarding the value of SGLT2i in SLE.
To cite this abstract in AMA style:
Petri M, Goldman D, Fava A, Magder L. Early Experience with SGLT2i in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/early-experience-with-sglt2i-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-experience-with-sglt2i-in-systemic-lupus-erythematosus/