Session Information
Date: Friday, November 6, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Clinical Manifestations
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: ANA testing as an approach to diagnosing and classifying SLE, now embedded in the EULAR/ACR Criteria, is more important than ever. Cross-sectional studies indicate that as few as 70% are ANA-positive, although ANA status may change over time. There are limited previous attempts to look at alterations in longitudinal ANA status in newly diagnosed SLE (1). We examined the dynamics of ANA tests in a longitudinal analysis using the SLICC Inception Cohort.
Methods: We evaluated demographic, clinical, and serological data from SLICC patients who fulfilled the 1997 Updated ACR SLE Classification Criteria and were within 15 months of diagnosis at enrolment. We performed two FDA-approved ANA tests: HEp-2 indirect immunofluorescence assay (IFA) and enzyme-linked immunosorbent assay (ELISA) (both from Inova Diagnostics, San Diego, CA) for each patient at enrolment and follow-up at years 3 and 5, at one central laboratory (Calgary, AB). A positive test was defined as a titer ≥1:80 for IFA and ≥20 units by ELISA. SLE-specific autoantibodies were performed using a commercially available autoantigen array (FIDIS Connective Profile-13, TheraDiag, Paris) in an addressable laser bead immunoassay. Anti-dsDNA positivity and titers were confirmed by chemiluminescent assay (BioFlash: Inova Diagnostics). Demographic and clinical characteristics of patients with a negative ANA test at any time point over the 5 years of disease were compared to patients with consistently positive ANA tests.
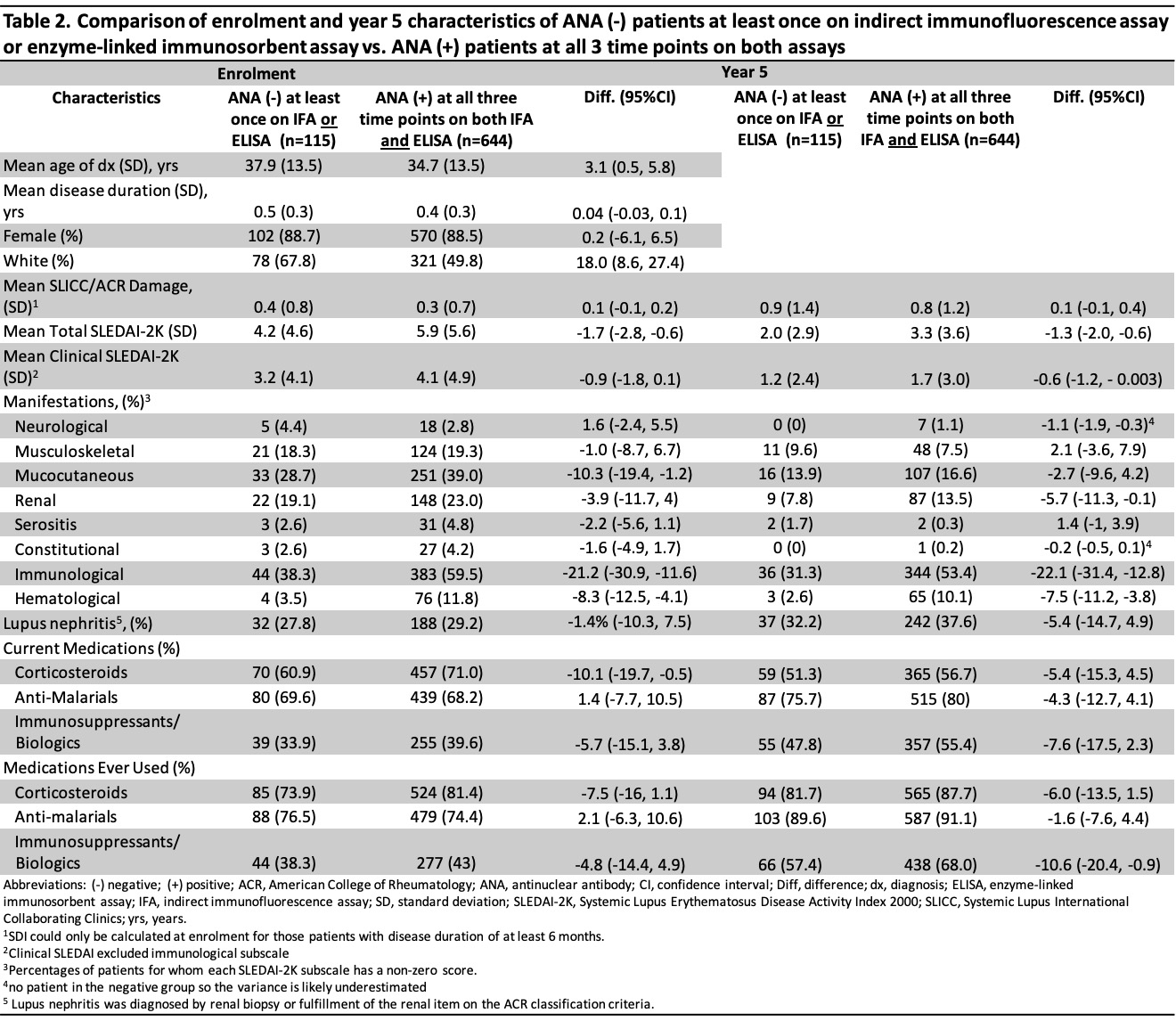
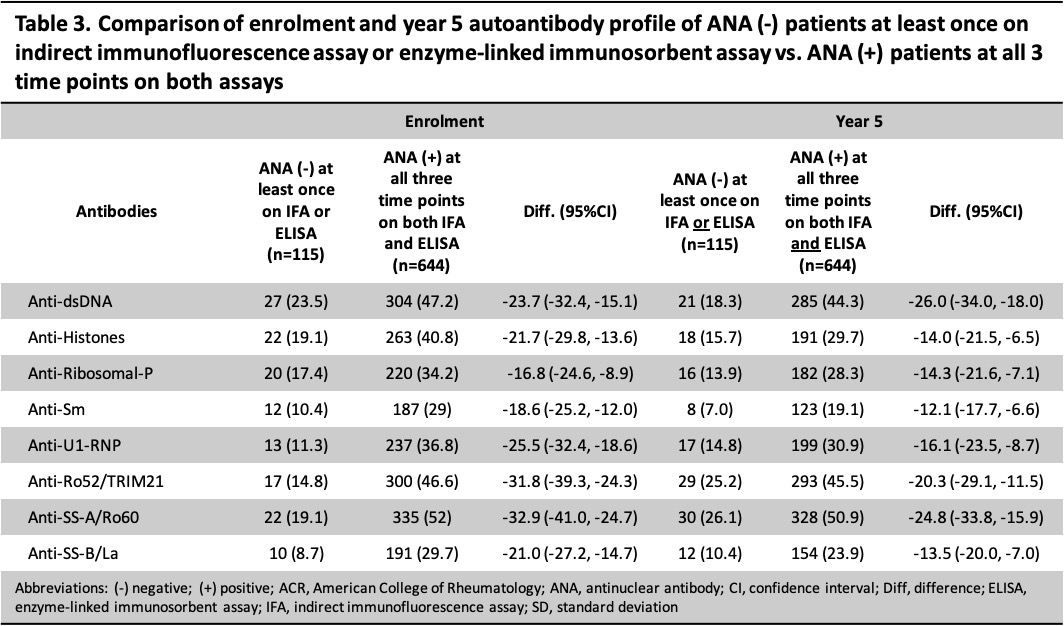
Results: Overall, 759 patients were included; 88.5% were female and the mean age at diagnosis was 35.2 (SD 13.5) years. There were 8 different ANA trajectories over 5 years; 29 (3.8%) tested negative at least once by ANA IFA and 101 (13.3%) by ELISA (Table 1). Compared to patients with a positive ANA at all 3 time points (n=644), those with any negative ANA (either IFA or ELISA, n=115) were diagnosed at an older age (37.9 vs 34.7 years), were more likely to be of white ethnicity (67.8% vs 49.8%), less likely to use steroids at enrolment (60.9% vs 71.0%), less likely to have ever used immunosuppressants/biologics at 5 years (57.4% vs 68.0%) (Table 2), and less likely to have other SLE-related antibodies at enrolment and year 5 (Table 3). The SLEDAI-2K of patients with a negative ANA (vs those with a persistently positive ANA) was lower (4.2 vs 5.9 at enrolment, 2.0 vs 3.3 at year 5) with less mucocutaneous (28.7% vs 39.0% at enrolment), immunological (38.3% vs 59.5% at enrolment and 31.3% vs 53.4% at year 5), and hematological (3.5% vs 11.8% at enrolment and 2.6% vs 10.1% at year 5) involvement.
Conclusion: Most of the 759 cases (84.8%) in the SLICC inception cohort remained ANA positive on both IFA and ELISA at each time point over the first 5 years of follow-up. The proportion of consistently ANA-positive patients was higher by IFA (96.2%) than ELISA (86.7%). Patients with at least one negative ANA over the first 5 years had features suggestive of milder disease (i.e. lower SLEDAI-2K, less steroid and immunosuppressive use, fewer autoantibodies) compared to patients with persistently positive ANAs, but differences in other disease features, e.g. nephritis and SLICC/ACR Damage Index, were not observed.
Ref: 1. Acosta-Merida A, Isenberg DA. CLIN EXP RHEUMATOL.2013;31(4):656
To cite this abstract in AMA style:
Choi M, Fritzler M, Costenbader K, Urowitz M, Hanly J, Gordon C, St. Pierre Y, Bae S, Romero-Díaz J, Sanchez-Guerrero F, Bernatsky S, Wallace D, Isenberg D, Rahman A, Merrill J, Fortin P, Gladman D, Bruce I, Petri M, Ginzler E, Dooley M, Ramsey-Goldman R, Manzi S, Jönsen A, Alarcón G, Van Vollenhoven R, Aranow C, Mackay M, Ruiz-Irastorza G, Lim S, Inanc M, Kalunian K, Jacobsen S, Peschken C, Kamen D, Askanase A, Clarke A. Dynamics of Anti-Nuclear Antibodies in a Longitudinal Study of a Large Systemic Lupus Erythematosus Cohort [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/dynamics-of-anti-nuclear-antibodies-in-a-longitudinal-study-of-a-large-systemic-lupus-erythematosus-cohort/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dynamics-of-anti-nuclear-antibodies-in-a-longitudinal-study-of-a-large-systemic-lupus-erythematosus-cohort/