Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
The Ontario Best Practices Research Initiative (OBRI) collects data on RA treatment in a real-world setting. Patients are enrolled and prospectively followed to assess response to biologic and DMARD therapy as well as to collect data on other factors. The objective of this study is to look at a real-world population and determine if initial disease activity influences the durability of the first used biologic in RA treatment.
Methods:
Biologic-naïve RA patients were included if they started a biologic at baseline or at any time after entry into OBRI. For initial DAS, we used the DAS28 value when it was measured between 6 months before and 3 months after the start of the first biologic, whichever was closer to the date of biologic use. This was done so as the initial DAS28 would best reflect the patient’s DAS at start of biologic. Patients were censored at treatment stop date or discontinuation date, date of death, or up to 18 months after initiation of biologic, whichever occurred first. Persistence was defined as the length of time the patients continued to receive the drug, irrespective of change in dose, route, or addition of any other DMARD, steroids, etc. If the drug was stopped for <60 days after which the patient restarted the same medication, it was considered a continuation and the duration was calculated accordingly. Survival was first compared using KM curves and then again using Cox-regression analysis. Analysis was performed for all years and also censored at 1.5 years.
Results:
471 patients were included. At 1 year, the survival probability was 0.76 (95% CI 0.68-0.81). Median survival was 5.005 years (95% CI 3.466-8.337). Patients who were on biologic monotherapy, with no concomitant DMARD use, has worse persistence of their initial biologic.
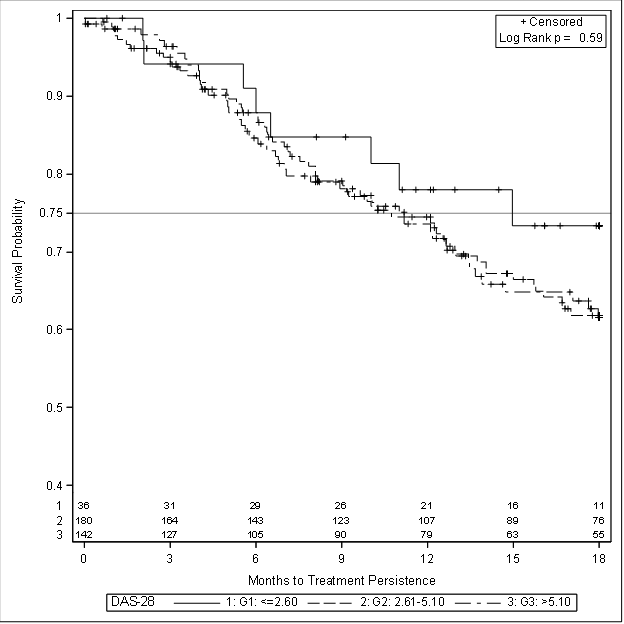
Patients were divided into three groups for analysis based on initial DAS28 score: remission (≤ 2.60), medium to high DAS28 (2.61-5.10) and severe (>5.10). Figure 1 shows the KM Plot of survival on biologic stratified by DAS28.
Despite the initial trend towards better survival associated with lower initial DAS, this was not statistically significant. Similarly, type of insurance (public/private) did not impact survival. As seen in other studies, the only significant factor to impact survival on initial biologic was use of DMARD with the biologic.
Conclusion:
Early/initial DAS28 score did not impact persistence on their initial biologic, nor did insurance type. This suggests that initial DAS28 score does not influence the durability of the initial biologic Combination of DMARD and biologic was more durable than biologic monotherapy.
Disclosure:
G. Rohekar,
None;
B. Jacob,
None;
J. E. Pope,
None;
C. Bombardier,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/durability-of-first-biologic-is-not-influenced-by-initialearly-das28/