Session Information
Date: Monday, October 27, 2025
Title: (1467–1516) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Laboratory monitoring for patients with systemic lupus erythematosus (SLE) is essential for assessing disease activity and detecting treatment-related adverse effects, including cytopenias. Neutropenia in particular may result from immunosuppressive medications. In patients with chronic neutropenia, providers may discontinue immunosuppressive medications out of concern for drug-induced myelosuppression. However, individuals with Duffy null (Fy[a–b–]) red blood cell phenotype, more common among those of African ancestry, have lower baseline absolute neutrophil counts without increased infection risk. The neutropenia associated with Duffy null phenotype may be misattributed to medication-related toxicity leading to inappropriate discontinuation of effective immunosuppressive therapy. We aimed to assess the frequency of Duffy antigen screening in SLE patients with neutropenia at an urban safety-net hospital and implemented a quality improvement (QI) intervention to increase screening.
Methods: We used Epic’s electronic health record (EHR) Slicer Dicer tool to identify patients with SLE and concurrent neutropenia at a single safety net hospital and reviewed the medical records to confirm presence of chronic neutropenia. We conducted an educational session for rheumatologists on the clinical relevance of the Duffy null phenotype and facilitated coordinated ordering of the test. We assessed whether a Duffy antigen screen had been performed and documented the results prior to and 2-months following the intervention.
Results: We identified 78 patients with SLE and leukopenia within the prior year, of whom 62 had neutropenia confirmed by medical record review. Prior to the intervention, 6 patients (9.7%) had undergone Duffy antigen testing, 5 of whom were Duffy null. Following the intervention, an additional 9 patients were screened over a 2-month period, increasing the post-intervention screening frequency to 14.5% (9/62). Among the 9 patients screened post-intervention, 7 had the Duffy null phenotype. Overall, 11 of the 12 patients identified as Duffy null were African American. (Table 1)
Conclusion: Our QI initiative led to a 4.8% absolute increase in Duffy antigen screening frequency in a 2-month post-intervention period. However, overall screening remained suboptimal. Given the higher prevalence of Duffy null phenotype in patients of African ancestry, which constitute a large percentage of SLE patients, recognizing this phenotype is important to prevent unnecessary medication changes that could compromise disease management. Future efforts to be considered include integrating electronic decision support tools, such as a Best Practice Advisory (BPA), to promote routine screening in SLE patients with chronic neutropenia. Our goal is to implement a more streamlined and automated process to ensure Duffy null antigen testing is routinely conducted.
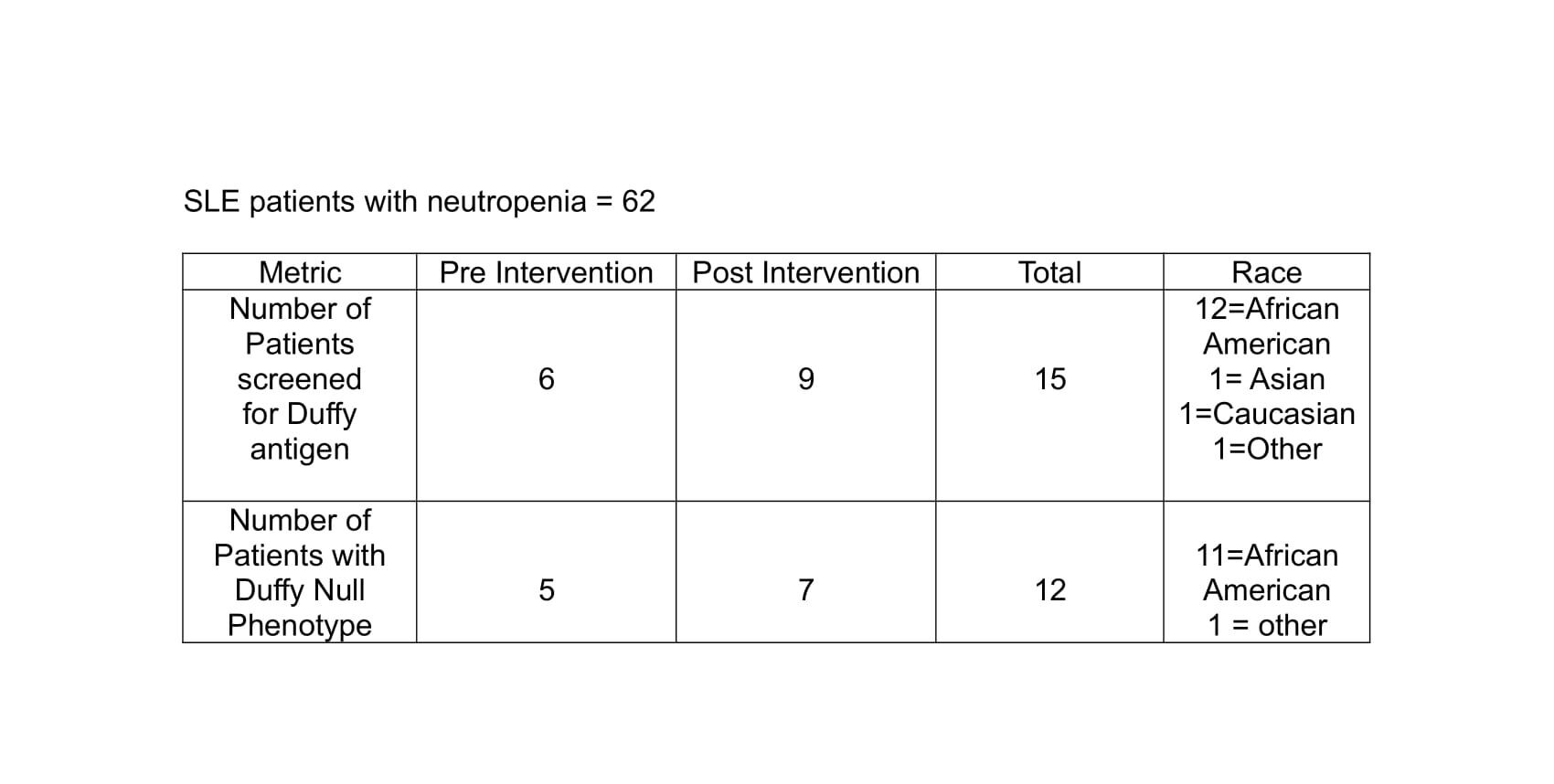 Table 1: Duffy Antigen Screening Outcomes in SLE Patients with Neutropenia
Table 1: Duffy Antigen Screening Outcomes in SLE Patients with Neutropenia
To cite this abstract in AMA style:
Sudireddy D, Yang J, Degirmenci H, Petrow E, York M. Duffy Antigen Screening in Systemic Lupus Erythematosus Patients with Neutropenia: A Quality Improvement Initiative [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/duffy-antigen-screening-in-systemic-lupus-erythematosus-patients-with-neutropenia-a-quality-improvement-initiative/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/duffy-antigen-screening-in-systemic-lupus-erythematosus-patients-with-neutropenia-a-quality-improvement-initiative/
